Abstract
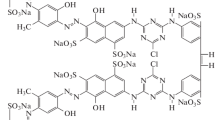
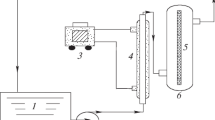
In this study, Ag/AgCl/TiO2 nanocomposites were synthesized by deposition–precipitation along photoreduction method and their photocatalytic performance was tested for removal of congo red under visible-light irradiation. Design of experiment with Taguchi method was employed to optimize synthesis parameters of composite such as the loading percentage of silver, the type of chlorine precursor, the duration of UV light radiation, the calcination temperature and the ratio of chloride to silver and also process parameters including photocatalyst dosage and dye concentration. According to results, dye concentration and type of chlorine precursor are significant factors. The optimal level of factors was precursor HCl, Cl/Ag ratio 2, silver loading 70%, 30 min exposure to UV light radiation, without calcination and photocatalyst dosage 700 ppm. The under optimum condition, high concentrations of congo red can be removed with high efficiency (96, 91 and 86% removal at concentration 30, 50 and 70 ppm, respectively). The synthesized nanocomposites were characterized by X-ray diffraction, scanning electron microscopy and UV–vis diffuse reflectance spectra.














Similar content being viewed by others
References
Chan, S.H.S.; Wu, T.Y.; Juan, J.C.; Teh, C.Y.: Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J. Chem. Technol. Biotechnol. 86, 1130–1158 (2011)
Bansal, P.; Singh, D.; Sud, D.: Photocatalytic degradation of azo dye in aqueous TiO2 suspension: Reaction pathway and identification of intermediates products by LC/MS. Sep. Purif. Technol. 72, 357–365 (2010)
Lin, W.C.; Yang, W.D.; Jheng, S.Y.: Photocatalytic degradation of dyes in water using porous nanocrystalline titanium dioxide. J. Taiwan Inst. Chem. Eng. 43, 269–274 (2012)
Daneshvar, N.; Khataee, A.; Rasoulifard, M.H.; Pourhassan, M.: Biodegradation of dye solution containing Malachite Green: optimization of effective parameters using Taguchi method. J. Hazard. Mater. 143, 214–219 (2007)
Kang, X.; Xia, Z.; Chen, R.; Sun, H.; Yang, W.: Effects of inorganic ions, organic polymers, and fly ashes on the sedimentation characteristics of kaolinite suspensions. Appl. Clay Sci. 181, 105220 (2019)
Rauf, M.; Meetani, M.; Hisaindee, S.: An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 276, 13–27 (2011)
Soutsas, K.; Karayannis, V.; Poulios, I.; Riga, A.; Ntampegliotis, K.; Spiliotis, X.; Papapolymerou, G.: Decolorization and degradation of reactive azo dyes via heterogeneous photocatalytic processes. Desalination 250, 345–350 (2010)
Umar, M.; Aziz, H.A.: Photocatalytic Degradation of Organic Pollutants in Water. Risk and Treatment. IntechOpen, London (2013)
Boutra, B.; Güy, N.; Özacar, M.; Trari, M.: Magnetically separable MnFe2O4/TA/ZnO nanocomposites for photocatalytic degradation of Congo Red under visible light. J. Magn. Magn. Mater. 497, 165994 (2020)
Zhang, Z.; Yates Jr., J.T.: Direct observation of surface-mediated electron–hole pair recombination in TiO2 (110). J. Phys. Chem. C 114, 3098–3101 (2010)
Zhang, Y.; Tang, Z.R.; Fu, X.; Xu, Y.J.: Nanocomposite of Ag–AgBr–TiO2 as a photoactive and durable catalyst for degradation of volatile organic compounds in the gas phase. Appl. Catal. B 106, 445–452 (2011)
Nakata, K.; Fujishima, A.: TiO2 photocatalysis: design and applications. J. Photochem. Photobiol. C 13, 169–189 (2012)
Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X.: Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances. Chem. Soc. Rev. 43, 5234–5244 (2014)
Zhou, J.; Cheng, Y.; Yu, J.: Preparation and characterization of visible-light-driven plasmonic photocatalyst Ag/AgCl/TiO2 nanocomposite thin films. J. Photochem. Photobiol. A. 223, 82–87 (2011)
Xu, H.; Li, H.; Xia, J.; Yin, S.; Luo, Z.; Liu, L.; Xu, L.: One-pot synthesis of visible-light-driven plasmonic photocatalyst Ag/AgCl in ionic liquid. ACS Appl. Mater. Interfaces 3, 22–29 (2010)
Liang, X.; Wang, P.; Li, M.; Zhang, Q.; Wang, Z.; Dai, Y.; Zhang, X.; Liu, Y.; Whangbo, M.H.; Huang, B.: Adsorption of gaseous ethylene via induced polarization on plasmonic photocatalyst Ag/AgCl/TiO2 and subsequent photodegradation. Appl. Catal. B 220, 356–361 (2018)
Liu, R.; Wang, P.; Wang, X.; Yu, H.; Yu, J.: UV-and visible-light photocatalytic activity of simultaneously deposited and doped Ag/Ag (I)–TiO2 photocatalyst. J. Phys. Chem. C 116, 17721–17728 (2012)
Liu, W.; Chen, D.; Yoo, S.H.; Cho, S.O.: Hierarchical visible-light-response Ag/AgCl@ TiO2 plasmonic photocatalysts for organic dye degradation. Nanotechnology 24, 405706 (2013)
Yang, Y.; Zhang, G.; Xu, W.: Facile synthesis and photocatalytic properties of AgAgClTiO2/rectorite composite. J. Colloid Interface Sci. 376, 217–223 (2012)
Edrissi, M.; Samadanian-Isfahani, S.; Soleymani, M.: Preparation of cobalt molybdate nanoparticles; Taguchi optimization and photocatalytic oxidation of Reactive Black 8 dye. Powder Technol. 249, 378–385 (2013)
Balak, Z.; Zakeri, M.; Rahimipour, M.; Salahi, E.: Taguchi design and hardness optimization of ZrB2-based composites reinforced with chopped carbon fiber and different additives and prepared by SPS. J. Alloys Compd. 639, 617–625 (2015)
Nakhostin-Panahi, P.; Salari, D.; Niaei, A.; Mousavi, S.M.: Study of M-ZSM-5 nanocatalysts (M: Cu, Mn, Fe, Co…) for selective catalytic reduction of NO with NH3: Process optimization by Taguchi method. Chin. J. Chem. Eng. 23, 1647–1654 (2015)
Nakhostin-Panahi, P.; Mohajer, SH.; Rasoulifard, M.H.; Farajmand, B.: Synthesis of Ag/AgCl/TiO2 nanocomposite and study of photocatalytic activity in VOCs removal from gas phase. Int. J. Environ. Anal. Chem. (2020). https://doi.org/10.1080/03067319.2020.1751146
Jie, H.; Jie, M.; Jiahua, M.; Huang, H.: Preparation of LaMnO3/graphene thin films and their photocatalytic activity. J. Rare Earths 328, 1126–1134 (2014)
Yin, H.; Wang, X.; Wang, L.; Nie, Q.; Zhang, Y.; Yuanet, Q.; Wu, W.: Ag/AgCl modified self-doped TiO2 hollow sphere with enhanced visible light photocatalytic activity. J. Alloys Compd. 657, 44–52 (2016)
Luo, L.; Li, Y.; Hou, J.; Yang, Y.: Visible photocatalysis and photostability of Ag3PO4 photocatalyst. Appl. Surf. Sci. 319, 332–338 (2014)
Ilyas, S.; Lee, J.C.; Bhatti, H.N.: One step bioleaching of sulphide ore with low concentration of arsenic by Aspergillus niger and Taguchi Orthogonal Array Optimization. Chin. J. Chem. Eng. 20, 923–929 (2012)
Varshosaz, J.; Eskandari, S.; Tabakhian, M.: Production and optimization of valproic acid nanostructured lipid carriers by the Taguchi design. Pharm. Dev. Technol. 15, 89–96 (2010)
Tian, B.; Dong, R.; Zhang, J.; Bao, S.; Yang, F.; Zhang, J.: Sandwich-structured AgCl@ Ag@ TiO2 with excellent visible-light photocatalytic activity for organic pollutant degradation and E. coli K12 inactivation. Appl. Catal. B. 158, 76–84 (2014)
Yang, Y.; Zhang, G.: Preparation and photocatalytic properties of visible light driven AgAgBr/attapulgite nanocomposite. Appl. Clay Sci. 67, 11–17 (2012)
Liu, J.; Wu, W.; Tian, Q.; Yang, S.; Sun, L.; Xiao, X.; Ren, F.; Jiang, C.; Roy, V.A.: Tube-like α-Fe2O3@ Ag/AgCl heterostructure: controllable synthesis and enhanced plasmonic photocatalytic activity. RSC Adv. 5, 61239–61248 (2015)
Yang, L.; Ma, X.; Zhang, W.; Gen, W.: Ag@AgCl-TiO2/organic rectorite/quaternize chitosan microspheres: an efficient and environmental photocatalyst. J. Appl. Poly. Sci. 134, 11 (2017)
Feng, Z.; Lv, X.; Wang, T.: TiO2 porous ceramic/Ag–AgCl composite for enhanced photocatalytic degradation of dyes under visible light irradiation. J. Porous Mater. 25, 189–198 (2018)
Acknowledgements
The authors would like to acknowledge the financial support from University of Zanjan and Iranian Nanotechnology Initiative.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakhostin Panahi, P., Mohajer, S. & Rasoulifard, M.H. Photocatalytic of Congo Red Decolorization in the Presence of Ag/AgCl/TiO2 Nanocomposite: Optimization of Process with Taguchi Method. Arab J Sci Eng 46, 5619–5632 (2021). https://doi.org/10.1007/s13369-020-05157-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-05157-3