Abstract
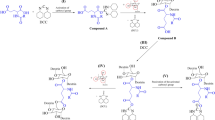
We are exploiting the use of a versatile catalyst taken from heterogeneous catalysis, i.e., ZrOCl2.8H2O to efficiently catalyze the reaction of dextran-succinate conjugate (Dex-SAn) with salicylic acid (SA) under homogeneous reaction conditions. Dextran was first linked with succinic anhydride using triethylamine as a base in DMAc to provide active functionalities (succinate moieties) situated away from the polymer chains. The resultant Dex-SAn conjugate was further esterified with SA using zirconium (IV) oxychloride octahydrate (ZrOCl2.8H2O) as a catalyst at 80 °C under N2. Reaction conditions and amount of catalyst ZrOCl2.8H2O and chiral support MCM-41 were optimized. This reaction methodology resulted in macromolecular prodrugs of SA as Dex-SAn-SA conjugates in good yield. The structures of Dex-SAn and newly synthesized Dex-SAn-SA conjugates were characterized using various spectroscopic techniques, i.e., FT-IR, 1H, and APT-13C NMR spectroscopy. The degree of substitution of SA on to Dex-SAn-SA was determined by UV/Vis spectroscopic methods. This reaction methodology can be modeled as a new protocol for facile attachment of several drug molecules onto the highly potential and biodegradable drug carrier dextran.







Similar content being viewed by others
References
Pospieszny, T.; Brycki, B.: Design and synthesis of new conjugates of bile acids with salicylic, acetylsalicylic and nicotinic acids. Lett. Org. Chem. 13, 302–309 (2016)
Steele, K.; Shirodaria, P.; O’Hare, M.; Merrett, J.D.; Irwin, W.G.; Simpson, D.I.H.; Pfister, H.: Monochloroacetic acid and 60% salicylic acid as a treatment for simple plantar warts: effectiveness and mode of action. Br. J. Dermatol. 118, 537–543 (1988)
Kratk, M.; Vinsova, J.; Buchta, V.: In Vitro antibacterial and antifungal activity of salicylanilide pyrazine-2-carboxylates. Med. Chem. 8, 732–741 (2012)
Dahlgren, M.K.; Kauppi, A.M.; Olsson, I.M.; Linusson, A.; Elofsson, M.: Design, synthesis, and multivariate quantitative structure-activity relationship of salicylanilides-potent inhibitors of type III secretion in Yersinia. J. Med. Chem. 50, 6177–6188 (2007)
Macielag, M.J.; Demers, J.P.; Fraga-Spano, S.A.; Hlasta, D.J.; Johnson, S.G.; Kanojia, R.M.; Russel, R.K.; Sui, Z.H.; Weidner-Wells, M.A.; Werblood, H.; Foleno, B.D.; Goldschmidt, R.M.; Loeloff, M.J.; Webb, G.C.; Barrett, J.F.: Substituted salicylanilides as inhibitors of two-component regulatory systems in bacteria. J. Med. Chem. 41, 2939–2945 (1998)
Alfonso, L.; Ai, G.; Spitale, R.C.; Bhat, G.J.: Molecular targets of aspirin and cancer prevention. Br. J. Cancer 111, 61–67 (2014)
Zhou, G.; Marathe, G.K.; Hartiala, J.; Hazen, S.L.; Allayee, H.; Tang, W.H.; McIntyre, T.M.: Aspirin hydrolysis in plasma is a variable function of butyrylcholinesterase and platelet-activating factor acetylhydrolase 1b2 (PAFAH1b2). J. Biol. Chem. 288, 11940–11948 (2013)
Hussain, M.A.; Abbas, K.; Jantan, I.; Bukhari, S.N.A.: Polysaccharide-based materials in macromolecular prodrug design and development. Int. Mater. Rev. 62, 78–98 (2017)
Hussain, M.A.; Abbas, K.; Amin, M.; Lodhi, B.A.; Iqbal, S.; Tahir, M.N.; Tremel, W.: Novel high-loaded, nanoparticulate and thermally stable macromolecular prodrug design of NSAIDs based on hydroxypropylcellulose. Cellulose 22, 461–471 (2015)
Sobczak, M.; Witkowska, E.; Olędzka, E.; Kolodziejski, W.: Synthesis and structural analysis of polyester prodrugs of norfloxacin. Molecules 13, 96–106 (2008)
Vyas, S.; Trived, P.; Chaturvedi, S.C.: Dextran-etodolac conjugates: synthesis, in vitro and in vivo evaluation. Acta Pol. Pharm. 66, 201–206 (2009)
Vyas, S.; Trived, P.; Chaturvedi, S.C.: Ketorolac-dextran conjugates: synthesis, in vitro and in vivo evaluation. Acta Pharm. 57, 441–450 (2007)
Hussain, M.A.; Hassan, Z.; Haseeb, M.T.; Iqbal, M.S.; Sher, M.; Tahir, M.N.; Tremel, W.; Bashir, S.; Ahmad, R.: Fabrication of potential macromolecular prodrugs of aspirin and diclofenac with dextran. Pak. J. Pharm. Sci. 24, 575–581 (2011)
Shrivastava, S.K.; Jain, D.K.; Trivedi, P.: Dextrans- potential polymeric drug carriers for flurbiprofen. Pharmazie 58, 389–391 (2003)
Shrivastava, S.K.; Jain, D.K.; Trivedi, P.: Dextrans-potential polymeric drug carriers for suprofen. Pharmazie 58, 804–806 (2003)
Vaidya, A.A.; Lele, B.S.; Kulkarni, M.G.; Mashelkar, R.A.: Enhancing ligand-protein binding in affinity thermoprecipitation: elucidation of spacer effects. Biotechnol. Bioeng. 64, 418–425 (1999)
McLeod, A.D.; Friend, D.R.; Tozera, T.N.: Synthesis and chemical stability of glucocorticoid-dextran esters: potential prodrugs for colon-specific delivery. Int. J. Pharm. 92, 105–114 (1993)
Goodlett, V.W.; Dougherty, J.T.; Patton, H.W.: Characterization of cellulose acetates by nuclear magnetic resonance. J. Polym. Sci. A: Polym. Chem. 9, 155–161 (1971)
Mantri, K.; Komura, K.; Sugi, Y.: ZrOCl2·8H2O catalysts for the esterification of long chain aliphatic carboxylic acids and alcohols. The enhancement of catalytic performance by supporting on ordered mesoporous silica. Green Chem. 7, 677–682 (2005)
Kirumakki, S.R.; Nagaraju, N.; Murthy, K.V.V.S.B.S.R.; Narayanan, S.: Esterification of salicylic acid over zeolites using dimethyl carbonate. Appl. Catal. A-Gen. 226, 175–182 (2002)
Heinze, T.; Liebert, T.; Katy, P.; Hussain, M.A.: Unconventional cellulose esters: synthesis, characterization and structure-property relations. Cellulose 10, 283–296 (2003)
Lee, H.Y.; Danjo, T.; Iwata, T.: Synthesis and characterization of dextrin derivatives by heterogeneous esterification. J. Polym. Res. 25, 183 (2018)
Larsen, C.: Macromolecular prodrugs. XII. Kinetics of release of naproxen from various polysaccharide ester prodrugs in neutral and alkaline solution. Int. J. Pharm. 51, 233–240 (1989)
Won, C.Y.; Chu, C.C.: Dextran-estrone conjugate: synthesis and in vitro release study. Carbohydr. Polym. 36, 327–334 (1988)
Draye, J.-P.; Delaey, B.; Van de Voorde, A.; Van den Bulcke, A.; Bogdanov, B.; Schacht, E.: In vitro release characteristics of bioactive molecules from dextran dialdehyde cross-linked gelatin hydrogel films. Biomaterials 19, 99–107 (1998)
Acknowledgment
Not applicable.
Funding
Not applicable
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Rights and permissions
About this article
Cite this article
Hussain, M.A., Iqbal, S., Ercisli, S. et al. Esterification of Salicylic acid with Succinylated Dextran Using ZrOCl2.8H2O over MCM-41: A Novel Strategy to Design Polysaccharide-Based Macromolecular Prodrugs. Arab J Sci Eng 46, 5583–5591 (2021). https://doi.org/10.1007/s13369-020-05143-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-05143-9