Abstract
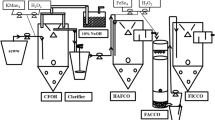
The treatment of lube oil processing industrial wastewater has been proposed by advanced oxidation processes such as Fenton and electro Fenton (EF) oxidation processes. The method of EF was further extended to homogeneous electro Fenton (Homo-EF) and heterogeneous electro Fenton (Hetero-EF) method of treatments. Three different types of anode materials were selected for the electrochemical oxidation against graphite as a cathode, i.e. SS304/graphite, graphite/graphite and Ti-MMO/graphite. Among the studied methods of treatment, the Hetero-EF performed by Ti-MMO/graphite electrode system showed maximum COD removal efficiency than the other electrode systems. The optimized conditions for the Hetero-EF by Ti-MMO/graphite were observed to be electrochemical oxidation time 120 min, solution pH 2.5, potential 7.5 V. Further, the instrumental analysis of UV–visible spectrophotometer confirmed the removal of organic concentration from lubricating oil processing wastewater.
Similar content being viewed by others
Abbreviations
- EF:
-
Electro Fenton
- Homo-EF:
-
Homogeneous electro Fenton
- Hetero-EF:
-
Heterogeneous electro Fenton
- SS304:
-
Stainless steel
- Ti-MMO:
-
Titanium doped mixed metal oxide
- AOP:
-
Advanced oxidation processes
- \(\hbox {COD}_{0}\) and \(\hbox {COD}_{{t}}\) :
-
Chemical oxygen demand at initial (\(t=0\)) and at time “t” min
- \(\hbox {K}_{\mathrm{app}}\) :
-
Apparent rate constant
- W :
-
Specific energy consumption
- V :
-
Average cell potential
- I :
-
Current (A)
- \(S_{\mathrm{v}}\) :
-
Sample volume in litres
- \(\Delta \)COD:
-
Difference in COD
References
Ahmed, A.F.; Ahmad, J.; Basma, Y.; Ramzi, T.: Assessment of alternative management techniques of tank bottom petroleum sludge in oman. J. Hazard. Mater. 141(3), 557–564 (2007). https://doi.org/10.1016/j.jhazmat.2006.07.023
Machın-Ramirez, C.; Okohc, A.I.; Morales, D.; Mayolo-Deloisa, K.; Quintero, R.; Trejo-Hernandez, M.R.: Slurry-phase biodegradation of weathered oily sludge waste. Chemosphere 70(4), 737–744 (2008). https://doi.org/10.1016/j.chemosphere.2007.06.017
Chen, G.H.; He, G.H.: Separation of water and oil from water-in-oil emulsion by freeze/thaw method. Sep. Purif. Technol. 31(1), 83–89 (2003). https://doi.org/10.1016/S1383-5866(02)00156-9
Bjarne, N.: Developments in membrane technology for water treatment. Desalination 153(1), 355–360 (2003). https://doi.org/10.1016/S0011-9164(02)01127-X
Zhong, J.; Sun, X.J.; Wang, C.L.: Treatment of oily wastewater produced from refinery processes using flocculation and ceramic membrane filtration. Sep. Purif. Technol. 32(1), 93–98 (2003). https://doi.org/10.1016/S1383-5866(03)00067-4
Benito, J.M.; Rios, G.; Ortea, E.; Fernandez, E.; Cambiella, A.; Pazos, C.; Coca, J.: Design and construction of a modular pilot for the treatment of oil-containing wastewaters. Desalination 147(1), 5–10 (2002). https://doi.org/10.1016/S0011-9164(02)00563-5
Li, Y.J.; Wang, F.; Zhou, G.D.: Aniline degradation by electrocatalytic oxidation. Chemosphere 53(10), 1229–1234 (2003). https://doi.org/10.1016/S0045-6535(03)00590-3
Koper, M.T.M.: Combining experiment and theory for understanding electrolysis. J. Electroanal. Chem. 574(2), 375–386 (2005). https://doi.org/10.1016/j.jelechem.2003.12.040
Santos, M.R.G.; Goulart, M.O.F.; Tonholo, J.; Zanta, C.L.P.S.: The application of electrochemical technology to the remediation of oily wastewater. Chemosphere 64(3), 393–399 (2006). https://doi.org/10.1016/j.chemosphere.2005.12.036
Hayat, S.; Ahmad, I.; Azam, Z.M.; Ahmad, A.; Inam, A.: Effect of long-term application of oil refinery wastewater on soil health with special reference to microbiological characteristics. Bioresour. Technol. 84(2), 159–163 (2002)
Ting, W.P.; Lu, M.C.; Huang, Y.H.: The reactor design and comparison of Fenton, electro-Fenton and photo-Fenton processes for mineralization of benzene and sulfonic acid (BSA). J. Hazard. Mater. 156(1), 421–427 (2008). https://doi.org/10.1016/j.jhazmat.2007.12.031
Lu, M.C.; Chang, Y.F.; Chen, I.M.; Huang, Y.Y.: Effect of chloride ions on oxidation of aniline by Fenton’s reagent. J. Environ. Manag. 75(2), 177–182 (2005). https://doi.org/10.1016/j.jenvman.2004.12.003
Qiang, Z.M.; Chang, J.H.; Huang, C.P.: Electrochemical generation of Fe\(^{2+}\) in Fenton oxidation processes. Water Res. 37(6), 1308–1319 (2003). https://doi.org/10.1016/S0043-1354(02)00461-X
Rosales, E.; Iglesias, O.; Pazos, M.; Sanroman, M.A.: Decolorisation of dyes under electro-Fenton process using Fe alginate gel beads. J. Hazard. Mater. 213(1), 369–377 (2012). https://doi.org/10.1016/j.jhazmat.2012.02.005
Peralta-Hernandez, J.M.; Meas-Vong, Y.; Rodriguez, F.J.; Chapman, T.W.; Maldonado, M.I.; Godinez, L.A.: In situ electrochemical and photo-electrochemical generation of the Fenton reagent: a potentially important new water treatment technology. Water Res. 40(9), 1754–1762 (2006). https://doi.org/10.1016/j.watres.2006.03.004
Duesterberg, C.K.; Waite, T.D.: A process optimization of Fenton oxidation using kinetic modeling. Environ. Sci. Technol. 40(13), 4189–4195 (2006). https://doi.org/10.1021/es060311
Brillas, E.; Sires, I.; Oturan, M.A.: Electro-Fenton process and related electrochemical technologies based Fenton’s chemistry. Chem. Rev. 109(12), 6570–6631 (2009). https://doi.org/10.1021/cr900136g
Sires, I.; Guivarch, E.; Oturan, N.; Oturan, M.A.: Efficient removal of triphenylmethane dyes from aqueous medium by in situ electro generated Fenton’s reagent at carbon-felt cathode. Chemosphere 72(4), 592–600 (2008). https://doi.org/10.1016/j.chemosphere.2008.03.010
Oturan, M.A.; Guivarch, E.; Oturan, N.; Sires, I.: Oxidation pathways of malachite green by Fe\(^{3+}\) catalyzed electro-Fenton process. Appl. Catal. B Environ. 82(3), 244–254 (2008). https://doi.org/10.1016/j.apcatb.2008.01.016
Quang, Z.; Chang, J.H.; Huang, C.P.: Electrochemical generation of hydrogen peroxide from dissolved oxygen in acidic solutions. Water Res. 36(1), 85–94 (2002). https://doi.org/10.1016/S0043-1354(01)00235-4
Duesterberg, C.K.; Mylon, S.E.; Waite, T.D.: ph effects on iron-catalyzed oxidation using Fentons reagent. Environ. Sci. Technol. 42(22), 8522–8527 (2008). https://doi.org/10.1021/es801720d
Bielski, B.H.J.; Allen, A.O.: Mechanism of the disproportionation of superoxide radicals. J. Phys. Chem. 81(11), 1048–1050 (1977). https://doi.org/10.1021/j100526a005
Chou, S.S.; Huang, Y.H.; Lee, S.N.; Huang, G.H.; Huang, C.P.: Treatment of high strength hexamine-containing wastewater by electro-Fenton method. Water Res. 33(3), 751–759 (1999). https://doi.org/10.1016/S0043-1354(98)00276-0
Moreno, A.D.; Frontana-Uribe, B.A.; Zamora, R.M.R.: Electro-Fenton as a feasible advanced treatment of process to produce reclaimed water. Water Sci. Technol. 50(2), 83–90 (2004)
Casado, J.; Fornaguera, J.; Galan, M.I.: Pilot scale mineralization of organic acids by electro-Fenton process plus sunlight exposure. Water Res. 40(13), 2511–2516 (2006). https://doi.org/10.1016/j.watres.2006.04.047
Ghoneim, M.M.; El-Desoky, H.S.; Zidan, N.M.: Electro-Fenton oxidation of sunset yellow FCF azo-dye in aqueous solution. Desalination 274(1), 22–30 (2011). https://doi.org/10.1016/j.desal.2011.01.062
Wang, C.T.; Hu, J.L.; Chou, W.L.; Kuo, Y.M.: Removal of color from real dye wastewater by electro-Fenton technology using a three-dimensional graphite electrode. J. Hazard. Mater. 152(2), 601–606 (2008). https://doi.org/10.1016/j.jhazmat.2007.07.023
Virkutyte, J.; Rokhina, E.; Jegatheesan, V.: Optimization of electro-Fenton denitrification of model wastewater using a response surface methodology. Bioresour. Technol. 101(5), 1440–1446 (2010). https://doi.org/10.1016/j.biortech.2009.10.041
Li, H.; Zhu, X.; Jiang, Y.; Ni, J.: Comparative electrochemical degradation of phthalic acid esters using boron-doped diamond and pt anodes. Chemosphere 80(8), 845–851 (2010). https://doi.org/10.1016/j.chemosphere.2010.06.006
Agladze, G.R.; Tsurtsumia, G.S.; Jung, B.I.; Kim, J.S.; Gorelishvili, G.: Comparative study of hydrogen peroxide electro-generation on gas-diffusion electrodes in undivided and membrane cells. J. Appl. Electrochem. 37(3), 375–383 (2007). https://doi.org/10.1007/s10800-006-9269-x
Wang, A.M.; Qu, J.H.; Ru, J.; Liu, H.J.; Ge, J.T.: Mineralization of an azo dye acid red 14 by electro-Fenton‘s reagent using a activated carbon fiber cathode. Dyes Pigments 65(3), 227–233 (2005). https://doi.org/10.1016/j.dyepig.2004.07.019
Ting, W.P.; Lu, M.C.; Huang, Y.H.: Kinetics of 2,6-dimethylamine degradation by electro-Fenton process. J. Hazard. Mater. 161(2), 1484–1490 (2009). https://doi.org/10.1016/j.jhazmat.2008.04.119
Gandini, D.; Mahe, E.; Michaud, P.A.; Haenni, W.; Perret, A.; Comninellis, C.: oxidation of carboxylic acids at boron-doped diamond electrodes for wastewater treatment. J. Appl. Electrochem. 30(12), 1345–1350 (2000). https://doi.org/10.1023/A:1026526729357
Shen, Z.M.; Wu, D.; Yang, J.; Yuan, T.; Wang, W.H.; Jia, J.P.: Methods to improve electrochemical treatment effect of dye wastewater. J. Hazard. Mater. 131(1), 90–97 (2006). https://doi.org/10.1016/j.jhazmat.2005.09.010
Korbahti, B.K.; Aktaş, N.; Tanyolac, A.: Optimization of electrochemical treatment of industrial paint wastewater with response surface methodology. J. Hazard. Mater. 148(1), 83–90 (2007). https://doi.org/10.1016/j.jhazmat.2007.02.005
Acknowledgements
The authors are thankful to The Head, Environment and Sustainability Department and the Director, CSIR-Institute of Minerals and Materials Technology for their encouragement and support. The authors also thank the Council of Scientific and Industrial Research, India, for the financial assistance by SETCA project (CSC-0113) to carry out the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boopathy, R., Das, T. New Approach of Integrated Advanced Oxidation Processes for the Treatment of Lube Oil Processing Wastewater. Arab J Sci Eng 43, 6229–6236 (2018). https://doi.org/10.1007/s13369-018-3417-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-018-3417-6