Abstract
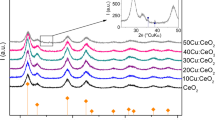
Pure, 3 mol% and 5 mol% Sr-doped cerium oxide nanoparticles were synthesized by facile aqueous co-precipitation method using cerium nitrate hexahydrate and strontium chloride hexahydrate as the precursors without using any capping agent. The synthesized material was characterized by XRD, SEM, EDX, TEM, Raman spectroscopy and UV–Vis spectroscopic techniques. SEM analysis showed agglomeration of the particles. The Debye–Scherrer analysis revealed fluorite structure of the synthesized material with crystallite size in 6–10 nm range. TEM confirmed the spherical morphology of the particles and particle size distribution in the range of 5–8 nm. UV–Vis spectroscopic study revealed that Sr-doping led to increase in the band gap from 3.2 to 3.7 eV and shifting of absorption edge to the lower wavelength. The blue shift in the band gap with the dopant concentration shows that the band gap of doped cerium oxide nanoparticles can be tuned with variation in the dopant concentration.
Similar content being viewed by others
References
Tok, A.I.Y.; Boey, F.Y.C.; Dong, Z.; Sun, X.L.: Hydrothermal synthesis of \(\text{ CeO }_{2}\) nano-particles. Mater. Process. Technol. 190, 217–222 (2007)
Marina, O.A.; Bagger, C.; Primdahl, S.; Mogensen, M.: Impedance of solid oxide fuel cell LSM/YSZ composite cathodes. Solid State Ionics 123, 199–208 (1999)
Trovarelli, A.; De Leitenburg, C.; Boaro, M.; Dolcetti, G.: The utilization of ceria in industrial catalysis. Catal. Today 50, 353–367 (1999)
Li, R.X.; Yabe, S.; Yamashita, M.; Momose, S.; Yoshida, S.; Yin, S.; Sato, T.: Synthesis and UV-shielding properties of ZnO- and CaO-doped \(\text{ CeO }_{2}\) via soft solution chemical process. Solid State Ionics 151, 235–241 (2002)
Masui, T.; Hirai, H.; Hamada, R.; Imanaka, N.; Adachi, G.; Sakatac, T.; Mori, H.: Synthesis and characterization of cerium oxide nanoparticles coated with turbostratic boron nitride. J. Mater. Chem. 13, 622–627 (2002)
Hu, C.; Zhang, Z.; Liu, H.; Gao, P.; Wang, Z.L.: Direct synthesis and structure characterization of ultrafine CeO2 nanoparticles. Nanotechnology 17, 5983–5987 (2006)
Araujo, V.D.; Avansi, W.; de Carvalho, H.B.; Moreira, M.L.; Longo, E.; Ribeiro, C.; Bernardi, M.I.B.: \(\text{ CeO }_{2}\) nanoparticles synthesized by a microwave-assisted hydrothermal method: evolution from nanospheres to nanorods. Cryst. Eng. Comm. 14, 1150–1154 (2012)
Liu, I.; Hon, M.H.; Teoh, L.G.: Structure and optical properties of CeO2 nanoparticles synthesized by precipitation. J. Electron. Mater. 42, 2536–2541 (2013)
Saranya, J.; Ranjith, K.S.; Saravanan, P.; Mangalaraj, D.; Kumar, R.T.R.: Cobalt-doped cerium oxide nanoparticles: enhanced photocatalytic activity under UV and visible light irradiation. Mater. Sci. Semicond. Process. 26, 218–224 (2014)
Mai, H.X.; Sun, L.D.; Zhang, Y.W.; Si, R.; Feng, W.; Zhang, H.P.; Liu, H.C.; Yan, C.H.: Shape-selective synthesis of oxygen storage behavior of ceria nanopolyhedra nanorods and nanocubes. J. Phys. Chem. B 109, 24380–24385 (2005)
Xufeng, G.; Chunlin, C.; Shiyuan, R.; Jian, Z.; Dangsheng, S.: Structural effects of cerium oxides on their thermal stability and catalytic performance in propane oxidation dehydrogenation. Chin. J. Catal. 33, 1059–1063 (2012)
Herrling, T.; Seifert, M.; Jung, K.: Cerium oxide: future UV filters in sunscreen. SOFW-J. 12, 10–14 (2013)
Goubin, F.; Rocquefelte, X.; Whangbo, M.H.; Montardi, Y.; Brec, R.; Jobic, S.: Experimental and theoretical characterization of the optical properties of \(\text{ CeO }_{2}\), \(\text{ SrCeO }_{3}\), and \(\text{ Sr }_{2}\text{ CeO }_{4}\) containing \(\text{ Ce }^{4+}\) \((\text{ f }^{0})\) ions. Chem. Mater. 16, 662–669 (2004)
Smijs, T.G.; Pavel, S.: Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 4, 95–112 (2011)
Minamidate, Y.; Yin, S.; Sato, T.: Synthesis and characterization of plate-like ceria particles for cosmetic application. Mater. Chem. Phys. 123, 516–520 (2010)
Prabaharan, D.M.D.M.; Sadaiyandi, K.; Mahendran, M.; Sagadevan, S.: Structural, optical, morphological and dielectric properties of cerium oxide nanoparticles. Mater. Res. 19(2), 478–482 (2016)
Farahmandjou, M.; Zarinkamar, M.; Firoozabadi, T.P.: Synthesis of cerium oxide \((\text{ CeO }_{2})\) nanoparticles using simple CO-precipitation method. Rev. Mex. Fis. E. 62, 496–499 (2016)
Liang, H.; Raitano, J.M.; He, G.; Akey, A.J.; Herman, I.P.; Zhang, L.; Chan, S.W.: Aqueous co-precipitation of Pd-doped cerium oxide nanoparticles: chemistry, structure, and particle growth. J. Mater. Sci. 42, 299–307 (2012)
Le Gal, A.; Abanades, S.: Dopant incorporation in ceria for enhanced water-splitting activity during solar thermochemical hydrogen generation. J. Phys. Chem. C 116(25), 13516–13523 (2012)
Kumar, A.; Babu, S.; Karakoti, A.S.; Schulte, A.; Seal, S.: Luminescence properties of europium-doped cerium oxide nanoparticles: role of vacancy and oxidation states. Langmuir 25, 10998–11007 (2009)
Ferreira, V.J.; Tavares, P.; Figueiredo, J.L.; Faria, J.L.: Effect of Mg, Ca and Sr on CeO2 based catalysts for oxidative coupling of methane: investigation on the oxygen species responsible for catalytic performance. Ind. Eng. Chem. Res. 51, 10535–10541 (2012)
Channei, D.; Inceesungvorn, B.; Wetchakun, N.; Ukritnukun, S.; Nattestad, A.: Photocatalytic degradation of methyl orange by \(\text{ CeO }_{2}\) and Fe-doped \(\text{ CeO }_{2}\) films under visible light irradiation. Sci. Rep. 4, 1–20 (2014)
Shirasaka, H.; Kisanuki, T.; Hirata, Y.; Matsunaga, N.; Sameshima, S.: Synthesis of gadolinium-doped ceria nanoparticles by electrolysis of aqueous solutions. J. Ceram. Process. Res. 14, 332–336 (2013)
Wang, H.F.; Li, H.Y.; Gong, X.Q.; Guo, Y.L.; Lu, G.Z.; Hu, P.: Oxygen vacancy formation in \(\text{ CeO }_{2}\) and \(\text{ Ce }_{(1--x)}\text{ Zr }_{(x)}\text{ O }_{2}\) solid solutions: electron localization, electrostatic potential and structural relaxation. Phys. Chem. Chem. Phys. 14(48), 16521–16535 (2012)
Manikantan, J.; Ramalingam, H.B.; Shekar, B.C.; Murugan, B.; Kumar, R.R.; Santhoshi, J.S.: Wide band gap of strontium doped hafnium oxide nanoparticles for optoelectronic device applications—synthesis and characterization. Mater. Lett. 186, 42–44 (2017)
Menon, A.S.; Kalarikkal, N.; Thomas, S.: Studies on structural and optical properties of ZnO and Mn- doped ZnO nanopowders. Ind. J. Nano. Sci. 1, 16–24 (2013)
Takeda, Y.; Mafuné, F.: Formation of wide bandgap cerium oxide nanoparticles by laser ablation in aqueous solution. Chem. Phys. Lett. 599, 110–115 (2014)
Yabe, S.; Yamashita, M.; Momose, S.; Yoshida, S.; Yin, S.; Sato, T.: UV-shielding properties of zinc oxide-doped ceria fine powders derived via soft solution chemical routes. Mater. Chem. Phys. 75(28), 39–44 (2002)
Ouhaibi, A.; Ghamnia, M.; Dahamni, M.A.; Heresanu, V.; Fauquet, C.; Tonneau, D.: The effect of strontium doping on structural and morphological properties of ZnO nanofilms synthesized by ultrasonic spray pyrolysis method. J. Sci. Adv. Mater. Devices 3, 29–36 (2018)
Jaiswal, N.; Singh, N.K.; Kumar, D.; Parkash, O.: Ceria co-doped with calcium (Ca) and strontium (Sr): a potential candidate as a solid electrolyte for intermediate temperature solid oxide fuel cells. J. Power Sour. 20, 45–54 (2014)
Matovic, B.; Bucevac, D.; Jiraborvornpongsa, N.; Yoshida, K.; Yano, T.: Synthesis and characterization of nanometric strontium-doped ceria solid solutions via glycine-nitrate procedure. J. Ceram. Soc. Jpn. 120(2), 69–73 (2012)
Ali, S.R.; Chandra, P.; Latwal, M.; Jain, S.K.; Bansal, V.K.: Growth of cadmium hexacyanidoferrate (III) nanocubes and its application in voltammetric determination of morphine. Bull. Chem. Soc. Jpn. 84(12), 1355–1361 (2011)
Ali, S.R.; Chandra, P.; Latwal, M.; Jain, S.K.; Bansal, V.K.; Singh, S.P.: Synthesis of nickel hexacyanoferrate nanoparticles and their potential as heterogeneous catalysts for the solvent-free oxidation of benzyl alcohol. Chin. J. Catal. 32(12), 1844–1849 (2011)
Liang, H.; Raitano, J.M.; He, G.; Akey, A.J.; Herman, I.P.; Zhang, L.; Chan, S.W.: Aqueous co-precipitation of Pd-doped cerium oxide nanoparticles: chemistry, structure, and particle growth. J. Mater. Sci. 47, 299–307 (2012)
Spanier, J.E.; Robinson, R.D.; Zhang, F.; Chan, S.W.; Herman, I.P.: Size-dependent properties of \(\text{ CeO }_{2-y}\) nanoparticles as studied by Raman scattering. Phys. Rev. B Condens. Matter. Mater. Phys. 64(2), 405–407 (2001)
Júnior, J.M.S.; Malta, L.F.B.; Garrido, F.M.S.; Ogasawara, T.; Medeiros, M.E.: Raman and Rietveld structural characterization of sintered alkaline earth doped ceria. Mater. Chem. Phys. 135, 957–964 (2012)
Araujo, V.D.; Avansi, W.; de Carvalho, H.B.; Moreira, M.L.; Longo, E.; Ribeiro, C.; Bernardi, M.I.B.: \(\text{ CeO }_{2}\) nanoparticles synthesized by a microwave-assisted hydrothermal method: evolution from nanospheres to nanorods. Cryst. Eng. Comm. 14, 1150–1154 (2012)
Labrincha, J.A.; Tobaldi, D.M.; Seabra, M.P.; Pullar, R.C.; Piccirillo, C.: W/Ag-co-doped titania nanopowders and their photocatalytic activity. Suranaree J. Sci. Technol. 20(3), 235–248 (2013)
Ramadoss, A.; Kim, S.J.: Synthesis and characterization of \(\text{ HfO }_{2}\) nanoparticles by sonochemical approach. J. Alloy. Compd. 544, 115–119 (2012)
Mott, N.F.; Davis, E.A.: Electronic Processes in Non-Crystalline Materials, 2nd edn. Clarendon Press, Oxford (1979)
Kim, D.T.; Yu, K.S.; Kim, W.T.; Kim, C.D.; Park, H.L.: Observation of Burstein–Moss shift in heavily copper-doped CaS:Cu phosphor. J. Mater. Sci. Lett. 11, 886–887 (1992)
Rekha, K.; Nirmala, M.; Manjula, G.; Anukaliani, A.: Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles. Phys. B. Phy. Cond. Mat. 405(15), 3180–3185 (2010)
Sharma, G.; Chawla, P.; Locha, S.P.; Singh, N.: Burstein Moss effect in nanocrystalline CaS: Ce. Bull. Mater. Sci. 34(4), 673–676 (2011)
Acknowledgements
The authors AK and AGS extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through research groups program under grant number RGP 1/49/39.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, S.R., Kumar, R., Kalam, A. et al. Effect of Strontium Doping on the Band Gap of \(\hbox {CeO}_{2}\) Nanoparticles Synthesized Using Facile Co-precipitation. Arab J Sci Eng 44, 6295–6302 (2019). https://doi.org/10.1007/s13369-018-03700-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-018-03700-x