Abstract
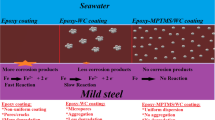
Epoxy-polyamide primer and coal tar epoxy topcoat system were tested to evaluate preservation of electrogalvanized mild steel (EGMS) under various naturally stimulated exposures including marine, industrial and urban test sites of Karachi city, while salt spray test was also conducted for comparison. Condition of coating was assessed by visual morphological inspection, gloss measurements, scanning electron microscopy (SEM), energy-dispersive X-ray (EDX) analysis and oxygen–carbon ratio techniques. Results indicated that the natural exposure testing (particularly at marine and industrial test sites) caused extremely high degradation of coal tar epoxy duplex coating systems as compared to accelerated testing. SEM has also revealed high degradation in the surface characteristics of the coating applied on EGMS after natural exposure testing. This is most likely due to the presence of several corrosive components of environment, emerging from the mist of salt spray in the atmosphere and pollution due to industry and automobiles. In addition to this, it was also found that the major modes of degradation were completely different in accelerated and natural exposure testing. Comparison of the results of coal tar epoxy coating systems applied on EGMS with our previous study on mild steel revealed high photodegradation of the coating systems applied on EGMS in natural exposure testing, i.e., oxygen/carbon ratios as determined by (EDX) analysis were higher for the coating systems applied on EGMS.

Similar content being viewed by others
References
Ilyas D.: Impedance measurements of polyester-coated galvanised mild steel in 10 × acid rainwater after an accelerated wet-dry test. Turk. J. Chem. 24, 239–246 (2000)
Thiraviyam P., Kannan K.: Inhibition of aminocyclohexane derivative on mild steel corrosion in 1N HCl. Arab. J. Sci. Eng. 38(7), 1757–1767 (2013)
Sadaf K., Quraishi M.A.: Synergistic effect of potassium iodide on inhibitive performance of thiadiazoles during corrosion of mild steel in 20 % sulfuric acid. Arab. J. Sci. Eng. 35(1), 71–82 (2010)
Yanjerappa A.N., Thimmappa V.V., Perdur V.N.: Electrodeposition of Zinc from Chloride Solution. Turk. J. Chem. 26, 725–734 (2002)
Mills D.J., Schaefer K.: Use of electrochemical methods to examine different surface preparation methods for organic coatings on steel. Prog. Org. Coat. 69, 193–198 (2010)
Yunan P., Ibrahim K., Wan N.W.B.: Effect of pH and chloride concentration on the corrosion of duplex stainless steel. Arab. J. Sci. Eng. 34(2), 115–127 (2009)
Shanthamma K.R., Thimmappa V.V.: Inhibition Studies of a Few Organic Compounds and Their Condensation Products on the Corrosion of Zinc in Hydrochloric Acid Medium. Turk. J. Chem. 27, 189–196 (2003)
Bano H., Khan M.I., Kazmi S.A.: Structure and Microstructure Studies of Epoxy Coating After Natural Exposure Testing. J. Chem. Soc. Pak. 33, 454–463 (2011)
Bano H., Khan M.I., Kazmi S.A.: SEM-EDX and FTIR Studies of Chlorinated Rubber Coating. J. Chem. Soc. Pak. 35, 95–108 (2013)
Bano H., Khan M. I., Kazmi S.A.: Studies in Accelerated (Predicted) Versus Observed Anticorrosive Performance for Coastal Industrialized Location. J. Chem. Soc. Pak. 35, 703–716 (2013)
SSPC-SP1.: Solvent Cleaning: Standard for the Removal of Surface Contaminants by the Use of Solvent. Pittsburgh, PA, USA (2004)
ASTM B-117.: Standard Practice for Operating Salt Spray (Fog) Apparatus, West Conshohocken, PA, USA (1997)
Akin A.: The salt spray corrosion of polymer coating on steel. Arab. J. Sci. Eng. 34(1), 139–145 (2009)
ISO 8565.: Metals and Alloys-Atmospheric Corrosion Testing: General Requirements for Field Tests. Geneva, Switzerland (1990)
EN ISO 4628-2.: Paints and Varnishes-Evaluation of Degradation of Coatings, Designation of Quantity and Size of Defects and of Intensity of Uniform Changes in Appearance, Part 2: Assessment of Degree of Blistering. CEN, Brussels (2003)
EN ISO 4628-3.: Paints and Varnishes-Evaluation of Degradation of Coatings, Designation of Quantity and Size of Defects and of Intensity of Uniform Changes in Appearance, Part 3: Assessment of Degree of Rusting. CEN, Brussels (2003)
EN ISO 4628-4.: Paints and Varnishes-Evaluation of Degradation of Coatings, Designation of Quantity and Size of Defects and of Intensity of Uniform Changes in Appearance, Part 4: Assessment of Degree of Cracking. CEN, Brussels (2003)
EN ISO 4628-10.: Paints and Varnishes-Evaluation of Degradation of Coatings, Designation of Quantity and Size of Defects and of Intensity of Uniform Changes in Appearance, Part 10: Evaluation of Filiform Corrosion. CEN, Brussels (2003)
EN ISO 2813.: Paints and Varnishes-Determination of Specular Gloss of Non-metallic Paint Films at 20°, 60° and 85°. CEN, Brussels (1999)
EN ISO 4628-8.: Paints and Varnishes-Evaluation of Degradation of Coatings, Designation of Quantity and Size of Defects and of Intensity of Uniform Changes in Appearance, Part 8: Evaluation of Delamination and Corrosion. CEN, Brussels (2003)
Ali N., Amir A.: Orthogonal Signal Correction—Partial Least Squares Method for Simultaneous Spectrophotometric Determination of Nickel, Cobalt, and Zinc. Turk. J. Chem. 32, 217–228 (2008)
EN ISO 4628-1.: Paints and Varnishes-Evaluation of Degradation of Coatings, Designation of Quantity and Size of Defects and of Intensity of Uniform Changes in Appearance, Part 1: General Introduction and Designation System. CEN, Brussels (2003)
Almeida E., Santos D., Fragata F., Fuente D., Morcillo M.: Anticorrosive painting for a wide spectrum of marine atmospheres: environmental-friendly versus traditional paint systems. Prog. Org. Coat. 57, 11–22 (2006)
Perrin F.X., Irigoyen M., Aragon E., Vernet J.L.: Evaluation of accelerated weathering tests for three paint systems: a comparative study of their aging behavior. Polym. Degrad. Stab. 72, 115–124 (2001)
Malshe V.C., Waghoo G.: Weathering study of epoxy paints. Prog. Org. Coat. 51, 267–272 (2004)
Babic R., Metikos H.M.: The study of coal tar epoxy protective coatings by impedance spectroscopy. Prog. Org. Coat. 23, 275–286 (1994)
Fragata F., Salai R.P., Amorim C., Almeida E.: Compatibility and incompatibility in anticorrosive painting: The particular case of maintenance painting. Prog. Org. Coat. 56, 257–268 (2006)
Yang X.F., Li J., Croll S.G., Tallman D.E., Bierwagen G.P.: Degradation of low gloss polyurethane aircraft coatings under UV and prohesion alternating exposures. Polym. Degrad. Stab. 80, 51–58 (2003)
Ocampo C., Armelin E., Liesa F., Aleman C., Ramis X., Iribarren J.I.: Application of a polythiophene derivative as anticorrosive additive for paints. Prog. Org. Coat. 53, 217–224 (2005)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bano, H., Mahmood, A., Khan, M.I. et al. Synergistic Corrosion Mitigation Appraisal of Coal Tar Epoxy Duplex Coating System by Spectroscopic and Microscopic Techniques. Arab J Sci Eng 39, 6783–6791 (2014). https://doi.org/10.1007/s13369-014-1236-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-014-1236-y