Abstract
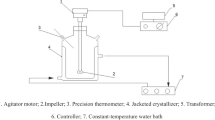
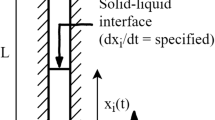
This paper covers the nucleation and growth of calcium carbonate crystals in a cooling crystallizer unit. In nucleation process, small particles are generated in a new phase inside the homogenous supersaturated phase, this situation occurs by cooling or warming. The system was modeled to assess the effect of temperature and mixer rotation on the size of produced crystals. Experiments were performed at 9 different temperatures and 5 different mixer rotations and the effect of these two parameters on the size of carbonate calcium crystals were studied. The results demonstrated that temperature decrease would lead to increase of the crystals diameter. Moreover, the increase in mixer rotation causes decrease in the crystals diameter. Based on these findings, the procedure of cooling the crystallizer was modeled in the meta-stable Region. Furthermore, optimum rotation of the mixer and the optimum measure of the seeds were calculated for the discrete cooling crystallizer. Finally, the model that defines the relation between the mixer rotation and the crystals’ diameter, the number of crystals and diameter of particles, as well as the number of particles and mixer rotation was determined to be developed.

Similar content being viewed by others
References
Falini G., Fermani S., Ripamonti A.: Crystallization of calcium carbonate salts into beta-chitin scaffold. J. Inorg. Biochem. 91, 475–480 (2002)
Garside, J.; Gaska, C.; Mullin, J.W.: Crystal growth rate studies with potas-siumsulphate in a fluidized bed crystallizer. J. Cryst. Growth 13/14, 510–516 (1972)
Mullin, J.W.; Nývlt, J.: Programmed cooling of batch crystallizers. Chem. Eng. Sci. 26, 369–377. doi:10.1016/0009-2509(71)83012-9 (1971)
Falini, G.; Gazzano, M.; Ripamonti, A.: Control of the architectural assembly of octacalciumPhospate crystals in denatured collagenous matrices. J. Mater. Chem. 10, 535–538 (2000)
Bernd, H.; Ehrenberg, H.; Marxen, J.C.; Becker, W.; Epple, M.: Calcium carbonate modifications in the mineralized shell of the freshwater snail biomphalariaglabrata. Chem. A Eur. J. 6, 3679–3685. doi:10.1002/1521 (2000)
Hou, W.; Feng, Q.: Simple method to control polymorphs of calcium carbonate crystals in CO2-diffusion precipitation. J. Cryst. Growth 282, 214–219 (2005)
Sherwin, M.B.; Shinnar, R.; Katz, S.: Dynamic behavior of the well-mixed isothermal crystallizer. AIChE J. 13, 1141–1154 (1967)
Hao, W.; Shenb, Q.; Zhaoa, Y.; Wanga, D.; Xua, D.F.: Crystallization habit of calcium carbonate in the presence of sodium dodecyl sulfate and/or polypyrrolidone. J. Cryst. Growth 260, 545–550 (2004)
Wheeler, A.P.; George, J. W.; Evans, C.A.: Control of CaCO3 nucleation and crystal growth by soluble matrix of oyster shell. Science 212, 1397–1398 (1981)
Loste, E.; Meldurm, F.C.: Control of calcium carbonate morphology by transformation of an amorphous precursor in a constrained volume. Chem. Commun. 10, 901–902 (2001)
Roque, J.; Molera, J.; Vendrell-Saz, M.; Salvadó, N.: Crystal size distribution of induced calcium carbonate crystals in polyaspartic acid and Mytilusedulis acidic organic proteins aqueous solutions. J. Cryst. Growth 262, 543–553 (2004)
Xie, A.; Jian, A.; Shen, Y.; Li, X.; Yuan, Z.: The role of Mg+2 and Mg+2/amino acid in controlling polymorph and morphology of calcium carbonate crystal. Mater. Chem. Phys. 101, 87–92 doi:10.1016/j.matchemphys.2006.02.019 (2006)
Guowei, Y.; Wang, L.; Huang, J.: Crystallization behavior of calcium carbonate in ethanol/water solution containing mixed nonionic/anionic surfactants. Powder Technol. 192, 58–64 (2009)
Chen P., Tai C., Lee K.C.: Morphology and growth rate of calcium carbonate crystals in a gas-liquid-solid reactive crystallizer. Chem. Eng. Sci. 52, 4171–4177 (1997)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Razmi, R., Hatampour, A. & Sedaghat, M.H. Modeling the Nucleation and Growth of Calcium Carbonate Crystals in Cooling Crystallizer Unit. Arab J Sci Eng 39, 15–22 (2014). https://doi.org/10.1007/s13369-013-0826-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-013-0826-4