Abstract
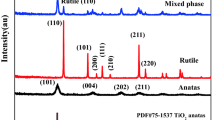
A nano-titanium dioxide (nano-TiO2) powder, with Zr (0.5–1 %) and Nb (0.5–1 %) impurities, is fabricated using a modified hydrothermal method, with low-grade mineral precursors. Samples were then sintered at 600, 800, and 1,000 °C, then analysed using XRD (crystallite size and phase conversion temperature) scanning electron microscope (SEM) (morphology), N2 adsorption–desorption isotherms (surface area), and UV–vis–NIR (absorbance and optical transmission) to study the effects of sintering on the structural and optical properties of the synthesized nanopowders. It was discovered that sintering to 1,000 °C reduces the surface area by 99 %, and increases the crystallite size by almost 2,000 %. Meanwhile, the phase conversion temperature of this sample is 33.3 % higher than that reported in the literature for 600 °C. SEM shows an extensive agglomeration and uneven distribution of nano-TiO2 particles before sintering. However, the sintered sample shows uniformity in particle size and distribution, and even though it is reduced significantly, agglomeration is still present. The absorbance of the samples is red-shifted towards the visible region (i.e., 380–700 nm), with the optical band-gap reduced by 10 %, when sintered to 1,000 °C. The optical transmission of nano-TiO2 is also reduced by 10 % when sintered to 1,000 °C, due to changes in its microstructure. It is therefore concluded that sintering nano-TiO2 improves its structural and optical properties, and paves the way for a multitude of novel applications.

Similar content being viewed by others
References
Negishi N., Takeuchi K., Ibusuki T.: The surface structure of titanium dioxide thin film photocatalyst. Appl. Surf. Sci. 121/122, 417–420 (1997)
Hussain, M.; Ceccarelli, R.; Marchisio, D.L.; Fino, D.; Russo, N.; Geobaldo, F.: Synthesis, characterization and photocatalytic application of novel TiO2 nanoparticles. Chem. Eng. J. 157, 45–51 (2010)
Kang, M.; Lee, S.-Y.; Chung, C.-H.; Cho, S.M.; Han, G.Y.; Kim, B.-W.; Yoon, K.J.: Characterization of a TiO2 photocatalyst synthesized by the solvothermal method and its catalytic performance for CHCl3 decomposition. J. Photoch. Photobio. A 144, 185–191 (2001)
Wu, L.; Yu, J.C.; Wang, X.; Zhang, L.; Yu, J.: Characterization of mesoporous nanocrystalline TiO2 photocatalysts synthesized via a sol solvothermal process at a low temperature. J. Solid State Chem. 178, 321–328 (2005)
Zhao, X.; Liu, M.; Zu, Y.: Fabrication of porous TiO2 film via hydrothermal method and its photocatalytic performances. Thin Solid Films. 515, 7127–7134 (2007)
Dong L., Cheng K., Weng W., Song C., Du P., Shen G., Han G.: Hydrothermal growth of rutile TiO2 nanorod films on titanium substrates. Thin Solid Films. 519, 4634–4640 (2011)
Karuppuchamy, S.; Nonomura, K.; Yoshida, T.; Sugiura, T.; Minoura, H.: Cathodic electrodeposition of oxide semiconductor thin films and their application to dye-sensitized solar cells. Solid State Ionics. 151 19–27 (2002)
Guo, W.; Lin, Z.; Wanga, X.; Song, G.: Sonochemical synthesis of nanocrystalline TiO2 by hydrolysis of titanium alkoxides. Microelectron. Eng 66, 95–101 (2003)
Chen X., Mao S.: Titanium dixoide nanomaterials: synthesis, properties, modifications and applications. Chem. Rev. 107, 2891–2959 (2007)
Hidaka, H.; Ajisaka, K.; Horikoshi, S.; Ogawa, T.; Takeuchi, K.; Zhao, J.; Serpone, N.: Comparative assessment of the efficiency of TiO2/OTE thin film electrodes fabricated by three deposition methods: photoelectrochemical degradation of the DBS anionic surfactant. J. Photochem. Photobio. A 138, 185–192 (2001)
Muniz, E.C.; Góes, M.S.; Silva, J.J.; Varela, J.A.; Joanni, E.; Parra, R.; Bueno, P.R.: Synthesis and characterization of mesoporous TiO2 nanostructured films prepared by a modified sol–gel method for application in dye solar cells. Ceram. Int. 37, 1017–1024 (2011)
Castro, A.L.; Nunes, M.R.; Carvalho, A.P.; Costa, F.M.; Florencio, M.H.: Synthesis of anatase TiO2 nanoparticles with high temperature stability and photocatalytic activity. Solid State Sci. 10, 602–606 (2008)
Swanepoel, R.: Determination of surface roughness and optical constants of inhomogeneous amorphous silicon films. J. Phys. E 17, 896 (1984)
Liu, Z.Y.; Sun, D.D.; Guo, P.; Leckie, J.O.: One-step fabrication and high photocatalytic activity of porous TiO2 hollow aggregates by using a low-temperature hydrothermal method without templates. Chem. Eur. J. 13, 1851–1855 (2007)
Estrellan C.R., Salim C., Hinode H.: Photocatalytic decomposition of perfluorooctanoic acid by iron and niobium co-doped titanium dioxide. J. Hazard. Mater. 179, 79–83 (2010)
Verma, A.; Basu, A.; Bakhshi, A.K.; Aghihotry, S.A.: Structural, optical and electrochemical properties of sol–gel derived TiO2 films: annealing effects. Solid State Ionics. 176, 2285–2295 (2005)
Ou, H.-H.; Lo, S.-L.: Review of titania nanotubes synthesized via the hydrothermal treatment: fabrication, modification, and application. Sep. Purif. Technol. 58, 179–191 (2007)
Li, Y.; Sun, X.; Li, H.; Wang, S.; Wei, Y.: Preparation of anatase TiO2 nanoparticles with high thermal stability and specific surface area by alcohothermal method. Powder Technol. 194, 149–152 (2009)
Jiang, Y.; Yin, H.; Sun, Y.; Liu, H.; Lei, L.; Chen, K.; Wada, Y.: Effects of organic acids on the size-controlled synthesis of rutile TiO2 nanorods. Appl. Surf. Sci. 253, 9277–9282 (2007)
Kim D.S., Kwak S.-Y.: The hydrothermal synthesis of mesoporous TiO2 with high crystallinity, thermal stability, large surface area, and enhanced photocatalytic activity. Appl. Catal. A-Gen. 323, 110–118 (2007)
Wang, Z.; Chen, C.; Wu, F.: Photodegradation of rhodamine B under visible light by bimetal codoped TiO2 nanocrystals. J. Hazard. Mater. 164, 615–620 (2009)
Zhang, Y.X.; Li, G.H.; Wu, Y.C.; Luo, Y.Y.; Zhang, L.D.: The formation of mesoporous TiO2 spheres via a facile chemical process. J. Phys. Chem. B 109, 5478–5484 (2005)
Mu, R.; Xu, Z.; Li, L.; Shao, Y.; Wan, H.; Zheng, S.: On the photocatalytic properties of elongated TiO2 nanoparticles for phenol degradation and Cr(VI) reduction. J. Hazard. Mater. 176, 495–502 (2010)
Fen L.B., Han T.K., Nee N.M., Ang B.C., Johan M.R.: Physico-chemical properties of titania nanotubes synthesized via hydrothermal and annealing treatment. Appl. Surf. Sci. 258, 431–435 (2011)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahdi, E.M., Hamdi, M. & Meor Yusoff, M.S. The Effect of Sintering on the Physical and Optical Properties of Nano-TiO2 Synthesized Via a Modified Hydrothermal Route. Arab J Sci Eng 38, 1701–1711 (2013). https://doi.org/10.1007/s13369-012-0384-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-012-0384-1