Abstract
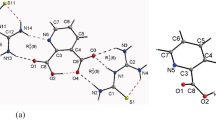
The optimized geometric parameters and vibrational frequencies of melaminium salt: 2,4,6-triamino-1,3,5-triazin-1,3-ium tartrate monohydrate in the ground state have been calculated by using the Hartree-Fock (HF) and density functional method (B3LYP) with 6-31G(d) and 6-31++G(d,p) basis sets. The results of the optimized molecular structure are presented and compared with the experimental X-ray diffraction. The molecule contains the weak hydrogen bonds of N–H. . .O and O–H. . .O types. The computed vibrational frequencies were used to determine the types of molecular motions associated with each of the experimental bands observed. The percentage weightage of internal coordinates contribution to the frequencies are given.

Similar content being viewed by others
References
Marchewka M.K., Baran J., Pietraszko A., Haznar A., Debrus S., Ratajczak H.: Crystal structure, vibrational spectra and nonlinear optical properties of new melaminium salt: 2,4,6-triamino-1,3,5-triazin-1,3-ium tartrate monohydrate. Solid State Sci. 5, 509–518 (2003)
Ratajczak H., Barycki J., Pietraszko A., Baran J., Debrus S., May M., Venturini J.: Preparation and structural study of a novel nonlinear molecularmaterial: the L-histidinum dihydrogenarsenate orthoarsenic acid crystal. J. Mol. Struct. 526, 269–278 (2000)
Ratajczak H., Debrus S., May M., Barycki J., Baran J.: Hydrogen-bonded organic solids with nonlinear optical properties. Bull. Pol. Acad. Sci. Chem. 48, 189–192 (2000)
Ratajczak H., Baran J., Barycki J., Debrus S., May M., Pietraszko A., Ratajczak H.M., Tramer A., Venturini J.: New hydrogen-bonded molecular crystals with nonlinear second-order optical properties. J. Mol. Struct. 555, 149–158 (2000)
Debrus S., Ratajczak H., Venturini J., Pinçon N., Baran J., Barycki J., Glowiak T., Pietraszko A.: Novel nonlinear optical crystals of noncentrosymmetric structure based on hydrogen bonds interactions between organic and inorganic molecules. Synth. Met. 127, 99–104 (2002)
Ratajczak H., Debrus S., Jakubas R., Baran J.: Second harmonic generation properties of [CH3NH3]5Bi2Br11 ferroelectric crystal. Bull. Pol. Acad. Sci. Chem. 48, 193–194 (2000)
Janczak J., Perpétuo G.J.: Melaminium phthalate. Acta Cryst. C 57, 123–125 (2001)
Janczak J., Perpétuo G.J.: Melaminium chloride hemihydrate. Acta Cryst. C 57, 1120–1122 (2001)
Janczak J., Perpétuo G.J.: Bis (melaminium) sulfate dihydrate. Acta Cryst. C 57, 1431–1433 (2001)
Perpétuo G.J., Janczak J.: Melaminium acetate acetic acid solvate monohydrate. Acta Cryst. C 58, o112–o114 (2002)
Janczak J., Perpétuo G.J.: Bis (melaminium) DL-malate tetrahydrate. Acta Cryst. C 59, o349–o352 (2003)
Janczak J., Kubiak R.: Supramolecular hydrogen-bonded 1D arrangement in the crystals of 2,4-diamino-6-benzyl-1,3,5-triazine and 2,4-diamino-6-(4′-methylbenzyl)-1,3,5-triazine. J. Mol. Struct. 920, 75–81 (2009)
Forsyth D.A., Sebag A.B.: Computed 13C NMR chemical shifts via empirically scaled GIAO shieldings and molecular mechanics geometries. Conformation and configuration from 13C shifts. J. Am. Chem. Soc. 119, 9483–9494 (1997)
Ditchfield R., Hehre W.J., Pople J.A.: Self-consistent molecular-orbital methods. IX. An extended Gaussian? Type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 54, 724–728 (1971)
Clark T., Chandrasekhar J., Spitznagel G.W., Schleyer P.V.R.: Efficient diffuse function-augmented basis sets for Anion Calculations. III. The 3-21+G set for first-row elements, Li-F. J. Comput. Chem. 4, 294–301 (1983)
Frisch M.J., Pople J.A.: Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 80, 3265–3269 (1984)
Avcı D., Atalay Y.: Theoretical analysis of vibrational spectra and scaling-factor of 2-aryl-1,3,4-oxadiazole derivatives. Int. J. Quant. Chem. 109, 328–341 (2009)
Akai N., Katsumoto Y., Ohno K., Aida M.: Vibrational anharmonicity of acetic acid studied by matrix- isolation near-infrared spectroscopy and DFT calculation. Chem. Phys. Lett. 413, 367–372 (2005)
Yoshida H., Takeda K., Okamura J., Ehara A., Matsuura H.: A new approach to vibrational analysis of large molecules by density functional theory: wavenumber-linear scaling method. J. Phys. Chem. A 106, 3580–3586 (2002)
Frisch, A.; Dennington, R.D. II; Keith, T.A.; Millam, J.; Nielsen, A.B.; Holder, A.J.; Hiscocks, J.: Gauss view version 4.1 user manual. Gaussian Inc., Wallingford (2007)
Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A. Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; Millam, J.M.; Iyengar, S.S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G.A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J.E.; Hratchian, H.P.; Cross, J.B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R.E.; Yazyev, O.; Austin, A.J.; Cami, R.; Pomelli, C.; Ochterski, J.W.; Ayala, P.Y.; Morokuma, K.; Voth, G.A.; Salvador, P.; Dannenberg, J.J.; Zakrzewski, V.G.; Dapprich, S.; Daniels, A.D.; Strain, M.C.; Farkas, O.; Malick, D.K.; Rabuck, A.D.; Raghavachari, K.; Foresman, J.B.; Ortiz, J.V.; Cui, Q.; Baboul, A.G.; Clifford, S.; Cioslowski, J.; Stefanov, B.B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R.L.; Fox, D.J.; Keith, T.; Al-Laham, M.A.; Peng, C.Y.; Nanayakkara, A.; Challacombe, M.; Gill, P.M.W.; Johnson, B.; Chen, W.; Wong, M.W.; Gonzalez, C.; Pople, J.A.: Gaussian 03, Revision E.01. Gaussian, Inc., Wallingford (2004)
Zerkowski J.A., McDonald J.C., Whitesides G.M.: Investigations into the robustness of hydrogen-bonded crystalline tapes. Chem. Mater. 6, 1250–1257 (1994)
Zerkowski J.A., Whitesides G.M.: Steric control of secondary, solid-state architecture in 1:1 complexes of melamines and barbiturates that crystallize as crinkled tapes. J. Am. Chem. Soc. 116, 4298–4304 (1994)
Janczak J., Perpetuo G.J.: Melaminium bis(4-hydroxybenzenesulfonate) dihydrate. Acta Cryst. C 57, 873–875 (2001)
Martin A., Pinkerton A.: Melaminium diperchlorate hydrate. Acta Cryst. C 51, 2174–2177 (1995)
Wang Y., Wei B., Wang Q.: Crystal structure of melamine cyanuric acid complex (1:1) trihydrochloride, MCA·3HCl. Crystallogr. Spectrosc. Res. 20, 79–84 (1990)
Scoponi M., Polo E., Pradella F., Bertolasi V., Carassiti V., Goberti P.: Crystal structure and spectroscopic analysis of melamine hydrobromide. Evidences of iso-melamine cations and charge-transfer complexes in solid state. J. Chem. Soc. Perkin Trans. 2, 1127–1132 (1992)
Kawasaki T., Kuroda Y., Nishikawa H.: The crystal structure of melamine diborate. J. Ceram. Soc. Jpn. 104, 935–938 (1996)
Atalay Y., Avcı D., Başoğlu A., Okur İ.: Molecular structure and vibrational spectra of melamine diborate by density functional theory and ab initio Hartree–Fock calculations. J. Mol. Struct. Theochem. 713, 21–26 (2005)
Atalay Y., Avcı D.: Theoretical studies of molecular structure and vibrational spectra of melaminium citrate, Spectrochim. Acta Part A 67, 327–333 (2007)
Tarcan E., Altındağ Ö., Avcı D., Atalay Y.: Molecular structure and vibrational assignment of melaminium phthalate by density functional theory (DFT) and ab initio Hartree–Fock (HF) calculations. Spectrochim. Acta Part A 71, 169–174 (2008)
Galabov B., Yamaguchi Y., Remington R.B., Schaefer H.F.: High level ab initio quantum mechanical predictions of infrared intensities. J. Phys. Chem. A 106, 819–832 (2002)
Scott A.P., Radom L.: Harmonic vibrational frequencies: an evaluation of Hartree–Fock, Møller–Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J. Phys. Chem. 100, 16502–16513 (1996)
Palafox M.A., Gill M., Nunez N.J., Rastogi V.K., Mittal L., Saharma R.: Scaling factors for the prediction of vibrational spectra. II. The aniline molecule and several derivatives. Int. J. Quant. Chem. 103, 394–421 (2005)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pekparlak, A., Avcı, D., Atalay, Y. et al. Theoretical Studies of Molecular Structure and Vibrational Spectra of Melaminium Salt: 2,4,6-Triamino-1,3,5-triazin-1,3-ium Tartrate Monohydrate. Arab J Sci Eng 37, 171–181 (2012). https://doi.org/10.1007/s13369-011-0151-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-011-0151-8