Abstract
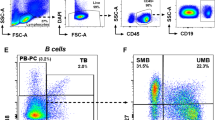
Multiple sclerosis (MS) is one of the common autoimmune diseases. The exact etiology of MS is still unclear, but recent studies have shown the possibility of infectious agent involvement such as Epstein-Barr virus (EBV) in MS pathophysiology. In this study, CD3 + CD8 + T cells of 25 new case MS patients were compared with healthy donors for expression of exhaustion marker, PD-1, using flow cytometry. Also, the expression of the EBV gene, BRCF-1, in PBMCs was analyzed using real-time PCR. Results revealed a lower frequency of CD3 + CD8 + T cells in MS patients. Also, increased expression of PD-1 was observed on CTLs which correlated with higher viral loads. Therefore, a lower frequency of CD8 + T cells but a higher exhaustion marker in MS patients reveals a new mechanism of EBV pathogenesis in MS development. The results suggest that inefficient immune control of EBV in patients with MS may cause exacerbation of the disease. Future studies on the mechanism of T cell exhaustion and chronic infections may aid in a better understanding of the disease and the design of effective therapies.



Similar content being viewed by others
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author.
References
Abdollahi M, Haghshenas M, Rafiei A, Abedini M (2016) Serologic evaluation of Epstein-Barr virus in multiple sclerosis. J Maz Univ Med Sci 26(140):71–77
Abrahamyan S, Abrahamyan S, Eberspächer B, Hoshi MM, Aly L, Luessi F et al (2020) Complete Epstein-Barr virus seropositivity in a large cohort of patients with early multiple sclerosis. J Neurol Neurosurg Psychiatry 91(7):681–686
Agostini S, Mancuso R, Guerini FR, Alfonso SD, Agliardi C, Hernis A et al (2018) HLA alleles modulate EBV viral load in multiple sclerosis. J Transl Med. 1–9. https://doi.org/10.1186/s12967-018-1450-6
Ascherio A, Munger KL, Lennette ET, Spiegelman D, Hernán MA, Olek MJ et al (2001) Epstein-Barr virus antibodies and risk of multiple sclerosis: a prospective study. J Am Med Assoc 286(24):3083–3088
Ayee R, Ofori MEO, Wright E, Quaye O (2020) Epstein Barr virus associated lymphomas and epithelia cancers in humans. J Cancer 11(7):1737–1750
Balfour HH, Sifakis F, Sliman JA, Knight JA, Schmeling DO, Thomas W (2013) Age-specific prevalence of Epstein-Barr virus infection among individuals aged 6–19 years in the United States and factors affecting its acquisition. J Infect Dis 208(8):1286–1293
Bar-Or A, Pender MP, Khanna R, Steinman L, Hartung HP, Maniar T, et al (2020) Epstein–Barr virus in multiple sclerosis: theory and emerging immunotherapies. Trends Mol Med. 26(3):296–310. https://doi.org/10.1016/j.molmed.2019.11.003
Bech E, Lycke J, Gadeberg P, Hansen H J, Malmeström C, Andersen O, ... Jakobsen J (2002) A randomized, double-blind, placebo-controlled MRI study of anti–herpes virus therapy in MS. Neurology 58(1):31–36
Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, Elledge SJ, Niebuhr DW, Scher AI, Munger KL, Ascherio A (2022) Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 375(6578):296–301. https://doi.org/10.1126/science.abj8222
Cencioni MT (2020) The immune regulation of PD-1/PDL-1 axis, a potential biomarker in multiple sclerosis. Neuroimmunol Neuroinflammation 2020:277–290
Cencioni MT, Magliozzi R, Nicholas R, Ali R, Malik O, Reynolds R, ... Muraro PA (2017) Programmed death 1 is highly expressed on CD 8+ CD 57+ T cells in patients with stable multiple sclerosis and inhibits their cytotoxic response to Epstein–Barr virus. Immunology 152(4):660–676. https://doi.org/10.1111/imm.12808
Cohen JI (2015) Epstein–barr virus vaccines. Clin Transl Immunol 4(1):e32
Draborg AH, Duus K. Houen G (2013) Epstein-Barr virus in systemic autoimmune diseases. Clin Dev Immunol 2013
Fernández-Menéndez S, Fernández-Morán M, Fernández-Vega I, Pérez-Álvarez A, Villafani-Echazú J. (2016) Epstein-Barr virus and multiple sclerosis. from evidence to therapeutic strategies. J Neurol Sci. 361(Review):213–9. https://doi.org/10.1016/j.jns.2016.01.013
Ferrari de Freitas L, de Melo Silva J, Nogueira Barbosa A, Miranda Santos E, Pinheiro-Silva, R, Soares Pontes G (2021) Epidemiological and liver biomarkers profile of epstein-barr virus infection and its coinfection with cytomegalovirus in patients with hematological diseases. Biomolecules 11(8):1151
Garcia CR, Jayswal R, Adams V, Anthony LB, Villano JL (2019) Multiple sclerosis outcomes after cancer immunotherapy. Clin Transl Oncol 21(10):1336–1342. https://doi.org/10.1007/s12094-019-02060-8
Guo J, Zhao C, Wu F, Tao L, Zhang C, Zhao, D, ... Yang K (2018) T follicular helper-like cells are involved in the pathogenesis of experimental autoimmune encephalomyelitis. Front Immunol 9:944
Holmøy T, Vartdal F (2004) Cerebrospinal fluid T cells from multiple sclerosis patients recognize autologous Epstein-Barr virus-transformed B cells. J Neurovirol 10(1):52–56
Holmøy T, Kvale EØ, Vartdal F (2004) Cerebrospinal fluid CD4+ T cells from a multiple sclerosis patient cross-recognize Epstein-Barr virus and myelin basic protein. J Neurovirol 10(5):278–283
Houen G, Trier NH, Frederiksen JL (2020) Epstein-Barr virus and multiple sclerosis. Front Immunol 11(December):1–11
Jadidi-Niaragh F, Mirshafiey A (2011) Th17 Cell, the new player of neuroinflammatory process in multiple sclerosis. Scand J Immunol 74(1):1–13
Jilek S, Schluep M, Harari A, Canales M, Lysandropoulos A, Zekeridou A et al (2012) HLA-B7–restricted EBV-specific CD8 + T cells are dysregulated in multiple sclerosis. J Immunol 188(9):4671–4680
Jin H-T, Anderson AC, Tan WG, West EE, Ha S-J, Araki K et al (2010) Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci. 107(33):14733–14738. http://www.pnas.org/cgi/doi/10.1073/pnas.1009731107
Jog NR, James JA (2021) Epstein Barr virus and autoimmune responses in systemic lupus erythematosus. Front Immunol 11(February):1–11
Kunkl M, Frascolla S, Amormino C, Volpe E, Tuosto L (2020) T Helper cells: the modulators of inflammation in multiple sclerosis. Cells 9(2):1–20
Kuri A, Jacobs BM, Jacobs BM, Vickaryous N, Pakpoor J, Middeldorp J et al (2020) Epidemiology of Epstein-Barr virus infection and infectious mononucleosis in the United Kingdom. BMC Public Health 20(1):1–9
Long HM, Meckiff BJ, Taylor GS (2019) The T-cell response to Epstein-Barr virus–new tricks from an old dog. Front Immunol 10(September):1–11
Lycke J, Malmestro C, Sta L (2003) Acyclovir Levels in Serum and Cerebrospinal Fluid after Oral Administration of Valacyclovir 47(8):2438–2441
Mody CH, Oykhman P (2010) Direct microbicidal activity of cytotoxic T-lymphocytes. J Biomed Biotechnol 2010(C)
Moser T, Akgün K, Proschmann U, Sellner J, Ziemssen T (2020) The role of TH17 cells in multiple sclerosis: therapeutic implications. Autoimmun Rev. 19(10):102647. https://doi.org/10.1016/j.autrev.2020.102647
Mouat IC, Morse ZJ, Shanina I, Brown KL, Horwitz MS (2021) Latent gammaherpesvirus exacerbates arthritis through modification of age-associated b cells. Elife 10:1–3
Pakpoor J, Giovannoni G (2013) Epstein–Barr virus and multiple sclerosis : association or causation? 287–97
Pender MP (2003) Infection of autoreactive B lymphocytes with EBV, causing chronic autoimmune diseases. Trends Immunol 24(11):584–588
Pender MP, Csurhes PA, Pfluger CMM, Burrows SR (2014) Deficiency of CD8+ effector memory T cells is an early and persistent feature of multiple sclerosis. Mult Scler J 20(14):1825–1832
Pender MP, Csurhes PA, Burrows JM, Burrows SR (2017) Defective T-cell control of Epstein-Barr virus infection in multiple sclerosis. Clin Transl Immunol. 6(1):e126. https://pubmed.ncbi.nlm.nih.gov/28197337/
Pender MP, Csurhes PA, Pfluger CM, Burrows SR (2011) Decreased CD8+ T cell response to Epstein-Barr virus infected B cells in multiple sclerosis is not due to decreased HLA class I expression on B cells or monocytes. BMC Neurology 11(1):1–6
Pender MP, Csurhes PA, Pfluger CM, Burrows SR (2012) CD8 T cell deficiency impairs control of Epstein–Barr virus and worsens with age in multiple sclerosis. J Neurol Neurosurg Psychiatry 83(3):353–354
Pender, M. P. (2011). The essential role of Epstein-Barr virus in the pathogenesis of multiple sclerosis. The Neuroscientist, 17(4), 351–367. https://doi.org/10.1177/1073858410381531
Pender MP (2012) CD8+ t-cell deficiency, Epstein-Barr virus infection, vitamin d deficiency, and steps to autoimmunity: a unifying hypothesis. Autoimmune Dis 1(1)
Perfetti V, Baldanti F, Lenti MV, Vanoli A, Biagi F, Gatti M et al (2016) Detection of active Epstein–Barr virus infection in duodenal mucosa of patients with refractory celiac disease. Clin Gastroenterol Hepatol. 14(8):1216–20. https://doi.org/10.1016/j.cgh.2016.03.022
Rostgaard K, Balfour HH, Jarrett R, Erikstrup C, Pedersen O, Ullum H et al (2019) Primary Epstein-Barr virus infection with and without infectious mononucleosis. PLoS ONE 14(12):1–14
Saeidi A, Zandi K, Cheok YY, Saeidi H, Wong WF, Lee CYQ et al (2018) T-cell exhaustion in chronic infections: reversing the state of exhaustion and reinvigorating optimal protective immune responses. Front Immunol 9(NOV):1–12
Salek-ardakani S, Schoenberger SP (2013) T cell exhaustion : a means or an end ? Nat Publ Gr 14(6):531–3. https://doi.org/10.1038/ni.2619
Sandu I, Cerletti D, Claassen M, Oxenius A (2020) Exhausted CD8 + T cells exhibit low and chronic LCMV infection. Nat Commun.1–11. https://doi.org/10.1038/s41467-020-18256-4
Schafflick D, Xu CA, Hartlehnert M, Cole M, Schulte-Mecklenbeck A, Lautwein T et al (2020) Integrated single cell analysis of blood and cerebrospinal fluid leukocytes in multiple sclerosis. Nat Commun 11(1):1–14
Serafini B, Rosicarelli B, Veroni C, Mazzola GA, Aloisi F (2019) Epstein-Barr Virus-Specific CD8 T Cells Selectively Infiltrate the Brain in Multiple Sclerosis and Interact Locally with Virus-Infected Cells: Clue for a Virus-Driven Immunopathological Mechanism. J Virol 93(24):1–21
Taylor GS, Long HM, Brooks JM, Rickinson AB, Hislop AD (2015) The immunology of Epstein-Barr virus-induced disease. Annu Rev Immunol 33:787–821
Teymoori-Rad M, Sahraian MA, Mokhtariazad T, Nejati A, Mozdabadi RSK, Amiri MM et al (2021) Illuminating the in vitro effects of Epstein-Barr virus and vitamin D on immune response in multiple sclerosis patients. J Neurovirol. 27(2):260–71. https://doi.org/10.1007/s13365-021-00951-7
Vandenbroeck K (2012) Cytokine gene polymorphisms and human autoimmune disease in the era of genome-wide association studies. J Interf Cytokine Res 32(4):139–151
Wagner H, Munger KL, Ascherio A (2004) Plasma viral load of Epstein–Barr virus and risk of multiple sclerosis 833–4
Wherry EJ, Kurachi M (2016) Molecular and Cellular Insights into T Cell Exhaustion 15(8):486–499
Willis SN, Stadelmann C, Rodig SJ, Caron T, Gattenloehner S, Mallozzi SS et al (2009) Epstein-Barr virus infection is not a characteristic feature of multiple sclerosis brain. Brain 132(12):3318–3328
Funding
The authors received financial support for the research from Kerman University of Medical Sciences under the grant number 96000710.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Atefeh Najmadin, MMM, and Ladan Langroudi. The first draft of the manuscript was written by Atefeh Najmadini and Ladan Langroudi, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All the procedures in this study have been reviewed and approved by the ethical committee of Kerman University of Medical sciences (reference number IR.KMU.REC.1396.2230).
Consent to participate
Informed consent was obtained prior to performing the procedure from all participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Najmadini, A., Mohammadi, M.M., Langroudi, L. et al. Increased expression of PD-1 in CD8 + CD3 + T cells correlates with EBV viral load in MS patients. J. Neurovirol. 28, 497–504 (2022). https://doi.org/10.1007/s13365-022-01083-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-022-01083-2