Abstract
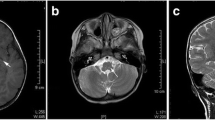
Enterovirus 71 (EV-A71) is a major causative agent for hand, foot, and mouth disease (HFMD), especially severe HFMD characterized by neurologic involvement. The objective of this study is to investigate the relationship between the distribution of neurologic infection and the outcomes of severe HFMD. A total of 139 suspected severe HFMD cases (92 were confirmed as EV-A71 infection) underwent clinical and laboratory diagnosis as well as magnetic resonance imaging (MRI) scans of the nervous system. Only those who were confirmed with EV-A71 infection were included in our study. The image data of severe EV-A71-related HFMD cases were retrospectively analyzed, and they were grouped according to lesion site location indicated by MRI. The distribution of lesions in the central nervous system shown by MRI indicated that there were 47 (51%) in brainstem, 33 (36%) in spinal nerve roots lower than T1 thoracic spine, four (5%) in brainstem plus cervical spinal cord involvement, three (3%) in cervical spinal cord, three (3%) in brainstem plus spinal nerve root lower than T1, and two (2%) in cervical and thoracic spinal cord lower than T1. Our analysis strongly substantiates the hypothesis of retrograde axonal transport (RAT) of EV-A71 pathogenesis, suggesting that the pharyngeal branch of the vagus nerve is a major route to the brainstem, and that ascending transportation via the spinal cord does not occur when spinal nerve roots are infected by EV-A71 via RAT.

ᅟ





Similar content being viewed by others
References
Alsop J, Flewett TH, Foster JR (1960) “Hand-foot-and-mouth disease” in Birmingham in 1959. Br Med J 2:1708–1711
Chen CS, Yao YC, Lin SC, Lee YP, Wang YF, Wang JR, Liu CC, Lei HY, Yu CK (2007) Retrograde axonal transport: a major transmission route of enterovirus 71 in mice. J Virol 81:8996–9003
Chen F, Li J, Liu T, Wang L, Li Y (2013) MRI characteristics of brainstem encephalitis in hand-foot-mouth disease induced by enterovirus type 71--will different MRI manifestations be helpful for prognosis? Eur J Paediatr Neurol 17:486–491
Chen F, Liu T, Li J, Xing Z, Huang S, Wen G (2014) MRI characteristics and follow-up findings in patients with neurological complications of enterovirus 71-related hand, foot, and mouth disease. Int J Clin Exp Med 7:2696–2704
Guan D, van der Sanden S, Zeng H, Li W, Zheng H, Ma C, Su J, Liu Z, Guo X, Zhang X, Liu L, Koopmans M, Ke C (2012) Population dynamics and genetic diversity of C4 strains of human enterovirus 71 in Mainland China, 1998-2010. PLoS One 7:e44386
Guerra AM, Waseem M (2017)[Updated 2018 Oct 27]. Hand foot and mouth disease. In: Treasure Island. StatPearls Publishing(Internet), FL. 2018 Jan
He Y, Ong KC, Gao Z, Zhao X, Anderson VM, McNutt MA, Wong KT, Lu M (2014) Tonsillar crypt epithelium is an important extra-central nervous system site for viral replication in EV71 encephalomyelitis. Am J Pathol 184:714–720
Ho M, Chen ER, Hsu KH, Twu SJ, Chen KT, Tsai SF, Wang JR, Shih SR (1999) An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med 341:929–935
Hu Y, Jiang L, Peng HL (2015) Clinical analysis of 134 children with nervous system damage caused by enterovirus 71 infection. Pediatr Infect Dis J 34:718–723
Jang S, Suh SI, Ha SM, Byeon JH, Eun BL, Lee YH, Seo HS, Eun SH, Seol HY (2012) Enterovirus 71-related encephalomyelitis: usual and unusual magnetic resonance imaging findings. Neuroradiology 54:239–245
Lee KY (2016) Enterovirus 71 infection and neurological complications. Korean J Pediatr 59:395–401
Lee DS, Lee YI, Ahn JB, Kim MJ, Kim JH, Kim NH, Hwang JH, Kim DW, Lee CG, Song TW (2015) Massive pulmonary hemorrhage in enterovirus 71-infected hand, foot, and mouth disease. Korean J Pediatr 58:112–115
Li J, Chen F, Liu T, Wang L (2012) MRI findings of neurological complications in hand-foot-mouth disease by enterovirus 71 infection. Int J Neurosci 122:338–344
Lian ZY, Li HH, Zhang B, Dong YH, Deng WX, Liu J, Luo XN, Huang B, Liang CH, Zhang SX (2017) Neuro-magnetic resonance imaging in hand, foot, and mouth disease: finding in 412 patients and prognostic features. J Comput Assist Tomogr 41:861–867
Liu HF, Won HS, Chung IH, Oh CS, Kim IB (2014) Variable composition of the internal and external branches of the accessory nerve. Clin Anat 27:97–101
Liu B, Luo L, Yan S, Wen T, Bai W, Li H, Zhang G, Lu X, Liu Y, He L (2015a) Clinical features for mild hand, foot and mouth disease in China. PLoS One 10:e0135503
Liu HF, Won HS, Chung IH, Kim IB, Han SH (2015b) Distribution of the internal branch of the human accessory nerve. Anat Sci Int 90:180–186
McMinn P, Stratov I, Nagarajan L, Davis S (2001) Neurological manifestations of enterovirus 71 infection in children during an outbreak of hand, foot, and mouth disease in Western Australia. Clin Infect Dis 32:236–242
Nagel MA, Gilden D (2014) Neurological complications of varicella zoster virus reactivation. Curr Opin Neurol 27:356–360
Ong KC, Badmanathan M, Devi S, Leong KL, Cardosa MJ, Wong KT (2008) Pathologic characterization of a murine model of human enterovirus 71 encephalomyelitis. J Neuropathol Exp Neurol 67:532–542
Ooi MH, Solomon T, Podin Y, Mohan A, Akin W, Yusuf MA, del Sel S, Kontol KM, Lai BF, Clear D, Chieng CH, Blake E, Perera D, Wong SC, Cardosa J (2007) Evaluation of different clinical sample types in diagnosis of human enterovirus 71-associated hand-foot-and-mouth disease. J Clin Microbiol 45:1858–1866
Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T (2010) Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol 9:1097–1105
Phyu WK, Ong KC, Kong CK, Alizan AK, Ramanujam TM, Wong KT (2017) Squamous epitheliotropism of Enterovirus A71 in human epidermis and oral mucosa. Sci Rep 7:45069
Prager P, Nolan M, Andrews IP, Williams GD (2003) Neurogenic pulmonary edema in enterovirus 71 encephalitis is not uniformly fatal but causes severe morbidity in survivors. Pediatr Crit Care Med 4:377–381
Shen WC, Chiu HH, Chow KC, Tsai CH (1999) MR imaging findings of enteroviral encephaloymelitis: an outbreak in Taiwan. AJNR Am J Neuroradiol 20:1889–1895
Singh S, Poh CL, Chow VT (2002) Complete sequence analyses of enterovirus 71 strains from fatal and non-fatal cases of the hand, foot and mouth disease outbreak in Singapore (2000). Microbiol Immunol 46:801–808
Skorzewska A, Bruska M, Wozniak W (1994) The development of the spinal accessory nerve in human embryos during 5th week (stages 14 and 15). Folia Morphol (Warsz) 53:177–184
Tan SH, Ong KC, Wong KT (2014) Enterovirus 71 can directly infect the brainstem via cranial nerves and infection can be ameliorated by passive immunization. J Neuropathol Exp Neurol 73:999–1008
Teoh HL, Mohammad SS, Britton PN, Kandula T, Lorentzos MS, Booy R, Jones CA, Rawlinson W, Ramachandran V, Rodriguez ML, Andrews PI, Dale RC, Farrar MA, Sampaio H (2016) Clinical characteristics and functional motor outcomes of enterovirus 71 neurological disease in children. JAMA Neurol 73:300–307
Tian H, Yang QZ, Liang J, Dong SY, Liu ZJ, Wang LX (2012) Clinical features and management outcomes of severe hand, foot and mouth disease. Med Princ Pract 21:355–359
Too IH, Yeo H, Sessions OM, Yan B, Libau EA, Howe JL, Lim ZQ, Suku-Maran S, Ong WY, Chua KB, Wong BS, Chow VT, Alonso S (2016) Enterovirus 71 infection of motor neuron-like NSC-34 cells undergoes a non-lytic exit pathway. Sci Rep 6:36983
Wang SM, Liu CC, Tseng HW, Wang JR, Huang CC, Chen YJ, Yang YJ, Lin SJ, Yeh TF (1999) Clinical spectrum of enterovirus 71 infection in children in southern Taiwan, with an emphasis on neurological complications. Clin Infect Dis 29:184–190
Wong KT, Munisamy B, Ong KC, Kojima H, Noriyo N, Chua KB, Ong BB, Nagashima K (2008) The distribution of inflammation and virus in human enterovirus 71 encephalomyelitis suggests possible viral spread by neural pathways. J Neuropathol Exp Neurol 67:162–169
Wong KT, Ng KY, Ong KC, Ng WF, Shankar SK, Mahadevan A, Radotra B, Su IJ, Lau G, Ling AE, Chan KP, Macorelles P, Vallet S, Cardosa MJ, Desai A, Ravi V, Nagata N, Shimizu H, Takasaki T (2012) Enterovirus 71 encephalomyelitis and Japanese encephalitis can be distinguished by topographic distribution of inflammation and specific intraneuronal detection of viral antigen and RNA. Neuropathol Appl Neurobiol 38:443–453
Xiu JH, Zhu H, Xu YF, Liu JN, Xia XZ, Zhang LF (2013) Necrotizing myositis causes restrictive hypoventilation in a mouse model for human enterovirus 71 infection. Virol J 10:215
Zeng H, Wen F, Huang W, Gan Y, Zeng W, Chen R, He Y, Wang Y, Liu Z, Liang C, Wong KK (2016) New findings, classification and long-term follow-up study based on MRI characterization of brainstem encephalitis induced by enterovirus 71. PLoS One 11:e0162877
Zerboni L, Ku CC, Jones CD, Zehnder JL, Arvin AM (2005) Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc Natl Acad Sci U S A 102:6490–6495
Acknowledgments
We thank Ann Turnley, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text draft of this manuscript.
Funding
This work was supported by the Science and Technology Planning Project of Guangdong Province, China (2017A020215071).
Author information
Authors and Affiliations
Contributions
HL designed and supervised the study. LS collected and analyzed data. TZ and FH collected and collated data. YY designed study and write the manuscript.
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, H., Su, L., Zhang, T. et al. MRI reveals segmental distribution of enterovirus lesions in the central nervous system: a probable clinical evidence of retrograde axonal transport of EV-A71. J. Neurovirol. 25, 354–362 (2019). https://doi.org/10.1007/s13365-019-00724-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-019-00724-3