Abstract
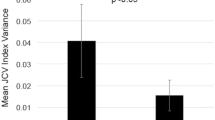
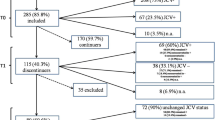
Sensitive biomarkers are needed to better manage multiple sclerosis (MS) patients for natalizumab (NTZ)-associated risk of progressive multifocal leukoencephalopathy (PML). A currently used risk stratification algorithm, mainly based on JC polyomavirus (JCPyV) serology, has not led to a reduction of PML incidence. Therefore, this study was designed to evaluate the presence and prevalence of JCPyV miRNAs in plasma of NTZ-treated MS patients, and to explore their biomarker potential for NTZ-associated PML risk assessment. Altogether, 102 plasma samples from 49 NTZ-treated and 28 interferon-beta (IFN-β)-treated relapsing-remitting MS patients, and 25 healthy controls (HCs) were analyzed for jcv-miR-J1-5p (5p miRNA) and jcv-miR-J1-3p (3p miRNA) expression. The overall detection rate of 5p miRNA was 84% (41/49) among NTZ-treated patients, 75% (21/28) among IFN-β-treated patients, and 92% (23/25) in HCs. Relative 5p miRNA expression levels were lower in NTZ-treated patients as compared to patients treated with IFN-β (p = 0.027) but not to HCs. Moreover, 5p miRNA expression inversely correlated with anti-JCPyV antibody index among JCPyV seropositive long-term NTZ-treated patients (r = −0.756; p = 0.002). The overall detection rate of 3p miRNA was low. Our results suggest that JCPyV miRNA in plasma may be linked to the reactivation of persistent JCPyV, to enhanced virus replication, and eventually to the risk of developing PML among NTZ-treated MS patients. However, further study is warranted in a larger data set including samples from PML patients to confirm the clinical relevance of JCPyV miRNA as a sign of/in viral reactivation, and to identify its potential to predict developing PML risk.


Similar content being viewed by others
References
Auvinen E (2017) Diagnostic and prognostic value of MicroRNA in viral diseases. Mol Diagn Ther 21:45–57
Basnyat P, Hagman S, Kolasa M, Koivisto K, Verkkoniemi-Ahola A, Airas L, Elovaara I (2015) Association between soluble L-selectin and anti-JCV antibodies in natalizumab-treated relapsing-remitting MS patients. Mult Scler Relat Disord 4:334–338
Bauman Y, Nachmani D, Vitenshtein A, Tsukerman P, Drayman N, Stern-Ginossar N, Lankry D, Gruda R, Mandelboim O (2011) An identical miRNA of the human JC and BK polyoma viruses targets the stress-induced ligand ULBP3 to escape immune elimination. Cell Host Microbe 9:93–102
Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, Lee S, Plavina T, Scanlon JV, Sandrock A, Bozic C (2012) Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 366:1870–1880
Broekema NM, Imperiale MJ (2013) miRNA regulation of BK polyomavirus replication during early infection. Proc Natl Acad Sci U S A 110:8200–8205
Chen CJ, Burke JM, Kincaid RP, Azarm KD, Mireles N, Butel JS, Sullivan CS (2014) Naturally arising strains of polyomaviruses with severely attenuated microRNA expression. J Virol 88:12683–12693
Chen Y, Bord E, Tompkins T, Miller J, Tan CS, Kinkel RP, Stein MC, Viscidi RP, Ngo LH, Koralnik IJ (2009) Asymptomatic reactivation of JC virus in patients treated with natalizumab. N Engl J Med 361:1067–1074
Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A (2010) Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol 9:438–446
Cutter GR, Stuve O (2014) Does risk stratification decrease the risk of natalizumab-associated PML? Where is the evidence? Mult Scler 20:1304–1305
Delbue S, Guerini FR, Mancuso R, Caputo D, Mazziotti R, Saresella M, Ferrante P (2007) JC virus viremia in interferon-beta -treated and untreated Italian multiple sclerosis patients and healthy controls. J Neuro-Oncol 13:73–77
Faulkner M (2015) Risk of progressive multifocal leukoencephalopathy in patients with multiple sclerosis. Expert Opin Drug Saf 14:1737–1748
Ferenczy MW, Marshall LJ, Nelson CD, Atwood WJ, Nath A, Khalili K, Major EO (2012) Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev 25:471–506
Gagne Brosseau MS, Stobbe G, Wundes A (2016) Natalizumab-related PML 2 weeks after negative anti-JCV antibody assay. Neurology 86:484–486
Giovannelli I, Martelli F, Repice A, Massacesi L, Azzi A, Giannecchini S (2015) Detection of JCPyV microRNA in blood and urine samples of multiple sclerosis patients under natalizumab therapy. J Neuro-Oncol 21:666–670
Jilek S, Mathias A, Canales M, Lysandropoulos A, Pantaleo G, Schluep M, Du Pasquier RA (2013) Natalizumab treatment alters the expression of T-cell trafficking marker LFA-1 alpha-chain (CD11a) in MS patients. Mult Scler 20:837–842
Jilek S, Jaquiery E, Hirsch HH, Lysandropoulos A, Canales M, Guignard L, Schluep M, Pantaleo G, Du Pasquier RA (2010) Immune responses to JC virus in patients with multiple sclerosis treated with natalizumab: a cross-sectional and longitudinal study. Lancet Neurol 9:264–272
Khalili K, White MK, Lublin F, Ferrante P, Berger JR (2007) Reactivation of JC virus and development of PML in patients with multiple sclerosis. Neurology 68:985–990
Kira J (2014) Disease concept, etiology and mechanisms of multiple sclerosis. Nihon Rinsho 72:1884–1894
Kolasa M, Hagman S, Verkkoniemi-Ahola A, Airas L, Koivisto K, Elovaara I (2016) Anti-JC virus seroprevalence in a Finnish MS cohort. Acta Neurol Scand 133:391–397
Kroh EM, Parkin RK, Mitchell PS, Tewari M (2010) Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods 50:298–301
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452
Lagatie O, Van Loy T, Tritsmans L, Stuyver LJ (2014) Viral miRNAs in plasma and urine divulge JC polyomavirus infection. Virol J 11:158–166
Lagatie O, Tritsmans L, Stuyver LJ (2013) The miRNA world of polyomaviruses. Virol J 10:268–288
Lee P, Plavina T, Castro A, Berman M, Jaiswal D, Rivas S, Schlain B, Subramanyam M (2013) A second-generation ELISA (STRATIFY JCV DxSelect) for detection of JC virus antibodies in human serum and plasma to support progressive multifocal leukoencephalopathy risk stratification. J Clin Virol 57:141–146
Link A, Balaguer F, Nagasaka T, Boland CR, Goel A (2014) MicroRNA miR-J1-5p as a potential biomarker for JC virus infection in the gastrointestinal tract. PLoS One 9:e100036
Lu S, Smith AP, Moore D, Lee NM (2010) Different real-time PCR systems yield different gene expression values. Mol Cell Probes 24:315–320
Martelli F, Giannecchini S (2017) Polyomavirus microRNAs circulating in biological fluids during viral persistence. Rev Med Virol 27:e1927
Munoz-Culla M, Irizar H, Castillo-Trivino T, Saenz-Cuesta M, Sepulveda L, Lopetegi I, Lopez de Munain A, Olascoaga J, Baranzini SE, Otaegui D (2014) Blood miRNA expression pattern is a possible risk marker for natalizumab-associated progressive multifocal leukoencephalopathy in multiple sclerosis patients. Mult Scler 20:1851–1859
Olsson T, Achiron A, Alfredsson L, Berger T, Brassat D, Chan A, Comi G, Eraksoy M, Hegen H, Hillert J, Jensen PE, Moiola L, Myhr KM, Oturai A, Schippling S, Siva A, Sorensen PS, Trampe AK, Weber T, Potts J, Plavina T, Paes D, Subramanyam M, Wiendl H, Dib H, Uren D, Hemmer B, Buck D (2013) Anti-JC virus antibody prevalence in a multinational multiple sclerosis cohort. Mult Scler 19:1533–1538
Pietila T, Nummi M, Auvinen P, Mannonen L, Auvinen E (2015) Expression of BKV and JCV encoded microRNA in human cerebrospinal fluid, plasma and urine. J Clin Virol 65:1–5
Plavina T, Subramanyam M, Bloomgren G, Richman S, Pace A, Lee S, Schlain B, Campagnolo D, Belachew S, Ticho B (2014) Anti-JCV antibody levels in serum or plasma further define risk of natalizumab-associated PML. Ann Neurol 76:802–812
Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW, Investigators AFFIRM (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354:899–910
Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O'Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald criteria". Ann Neurol 58:840–846
Rocca A, Martelli F, Delbue S, Ferrante P, Bartolozzi D, Azzi A, Giannecchini S (2015) The JCPYV DNA load inversely correlates with the viral microrna expression in blood and cerebrospinal fluid of patients at risk of PML. J Clin Virol 70:1–6
Rudick RA, O'Connor PW, Polman CH, Goodman AD, Ray SS, Griffith NM, Jurgensen SA, Gorelik L, Forrestal F, Sandrock AW, Goelz SE (2010) Assessment of JC virus DNA in blood and urine from natalizumab-treated patients. Ann Neurol 68:304–310
Schwab N, Schneider-Hohendorf T, Posevitz V, Breuer J, Gobel K, Windhagen S, Brochet B, Vermersch P, Lebrun-Frenay C, Posevitz-Fejfar A, Capra R, Imberti L, Straeten V, Haas J, Wildemann B, Havla J, Kumpfel T, Meinl I, Niessen K, Goelz S, Kleinschnitz C, Warnke C, Buck D, Gold R, Kieseier BC, Meuth SG, Foley J, Chan A, Brassat D, Wiendl H (2013) L-selectin is a possible biomarker for individual PML risk in natalizumab-treated MS patients. Neurology 81:865–871
Seo GJ, Fink LH, O'Hara B, Atwood WJ, Sullivan CS (2008) Evolutionarily conserved function of a viral microRNA. J Virol 82:9823–9828
Silvy M, Mancini J, Thirion X, Sigaux F, Gabert J (2005) Evaluation of real-time quantitative PCR machines for the monitoring of fusion gene transcripts using the Europe against cancer protocol. Leukemia 19:305–307
Wollebo HS, White MK, Gordon J, Berger JR, Khalili K (2015) Persistence and pathogenesis of the neurotropic polyomavirus JC. Ann Neurol 77:560–570
Acknowledgements
The authors thank Keijo Koivisto (Seinäjoki Central Hospital, Department of Neurology, Seinäjoki, Finland), Auli Verkkoniemi-Ahola (Department of Clinical Neurosciences, Helsinki University Central Hospital, Helsinki, Finland), and Laura Airas (Department of Clinical Neurosciences, Turku University hospital, Turku, Finland) for providing samples and clinical information on patients from their centers. The authors also thank Mika Helminen (School of Health Sciences, University of Tampere) for his advice in statistical analyses. The study was financially supported by the Tampere University Hospital Medical Fund, Pirkanmaa Regional Fund of the Finnish Cultural Foundation, Finnish MS Foundation, and Alfred Kordelin Foundation of Finland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors have no conflict of interests to declare.
Electronic supplementary material
Supplementary Table 1
(DOCX 45 kb)
Rights and permissions
About this article
Cite this article
Basnyat, P., Virtanen, E., Elovaara, I. et al. JCPyV microRNA in plasma inversely correlates with JCPyV seropositivity among long-term natalizumab-treated relapsing-remitting multiple sclerosis patients. J. Neurovirol. 23, 734–741 (2017). https://doi.org/10.1007/s13365-017-0560-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-017-0560-x