Abstract
The risk of developing progressive multifocal leukoencephalopathy (PML), as a consequence of infection/reactivation with JC virus (JCV), is consistent in natalizumab-treated multiple sclerosis (MS) patients, with 430 cases of PML reported so far. The risk of PML is higher in JCV seropositive patients, and it is recommended that only MS patients without JCV antibodies should be enrolled in the treatment postulating that they do not have JCV infection.
We have studied forty-two natalizumab-treated MS patients, and urine and blood were collected monthly for up to 60 months. JCV and BK virus (BKV) DNA presence was verified using quantitative real-time PCR assays, and serum anti-JCV antibodies were measured with the Stratify and/or Stratify DxSelect tests.
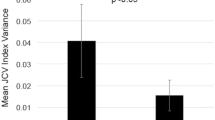
JCV and BKV DNA were not found in the blood samples, whereas they were found at least once in the urine of 21 of 42 (50 %) and of 25/42 (59.5 %) patients, respectively. JCV DNA urinary shedding increased up to month 24 of natalizumab treatment (45.2 %), and the effect of time was significant for JCV (p = 0.04), but not for BKV (p = 0.39). JCV viruria and seropositivity did not completely correlate, since three patients shedding JCV DNA in the urine were seronegative according to the serological tests.
The results indicated that natalizumab therapy may increase the rate of JCV urinary shedding. Additionally, we confirmed that the identification of JCV carriers cannot solely rely on serological tests, but sensitive methods for viral DNA detection should be adopted to more precisely identify the truly JCV uninfected cases.


Similar content being viewed by others
References
Berger JR, Khalili K (2012) The pathogenesis of progressive multifocal leukoencephalopathy. Discov Med 12:495–503
Berger JR, Houff SA, Gurwell J, Vega N, Miller CS, Danaher RJ (2013) JC virus antibody status underestimates infection rates. Ann Neurol 74:84–90
Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, Lee S, Plavina T, Scanlon JV, Sandrock A, Bozic C (2012) Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 366:1870–1880
Chen Y, Bord E, Tompkins T, Miller J, Tan CS, Kinkel RP, Stein MC, Viscidi RP, Ngo LH, Koralnik IJ (2009) Asymptomatic reactivation of JC virus in patients treated with natalizumab. N Engl J Med 361:1067–1074
Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A (2010) Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol 9:438–446
Delbue S, Sotgiu G, Fumagalli D, Valli M, Borghi E, Mancuso R, Marchioni E, Maserati R, Ferrante P (2005) A case of a PML patient with four different JC virus TCR rearrangements in CSF, blood, serum and urine. J Neurovirol 11:51–57
Delbue S, Guerini F, Mancuso R, Caputo D, Mazziotti R, Saresella M, Ferrante P (2007) JCV viremia in interferon-beta treated and untreated Italian multiple sclerosis patients and healthy controls. J Neurovirol 13:77–78
Derfuss T, Kappos L (2013) Predicting PML in natalizumab-treated patients: can we do better? J Neurol Neurosurg Psychiatry 84:1182–1183
Dominguez-Mozo MI, Garcia-Montojo M, De Las HV et al (2013) Anti-JCV antibodies detection and JCV DNA levels in PBMC, serum and urine in a cohort of Spanish multiple sclerosis patients treated with natalizumab. J NeuroImmune Pharm 8:1277–86
Dörries K, Vogel E, Günther S, Czub S (1994) Infection of human polyomaviruses JC and BK in peripheral blood leukocytes from immunocompetent individuals. Virology 198:59–70
Ferrante P, Caldarelli-Stefano R, Omodeo-Zorini E, Mediati M, Fainardi E, Granieri E, Caputo D (1997) Comprehensive investigation of the presence of JC virus in AIDS patients with and without progressive multifocal leukoencephalopathy. J Med Virol 52:235–242
Gorelik L, Lerner M, Bixler S, Crossman M, Schlain B, Simon K, Pace A, Cheung A, Chen LL, Berman M, Zein F, Wilson E, Yednock T, Sandrock A, Goelz SE, Subramanyam M (2010) Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol 68:295–303
Jilek S, Jaquiery E, Hirsch HH, Lysandropoulos A, Canales M, Guignard L, Schluep M, Pantaleo G, Du Pasquier RA (2010) Immune responses to JC virus in patients with multiple sclerosis treated with natalizumab: a cross-sectional and longitudinal study. Lancet Neurol 9:264–72
Kappos L, Bates D, Edan G, Eraksoy M, Garcia-Merino A, Grigoriadis N, Hartung HP, Havrdová E, Hillert J, Hohlfeld R, Kremenchutzky M, Lyon-Caen O, Miller A, Pozzilli C, Ravnborg M, Saida T, Sindic C, Vass K, Clifford DB, Hauser S, Major EO, O'Connor PW, Weiner HL, Clanet M, Gold R, Hirsch HH, Radü EW, Sørensen PS, King J (2011) Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol 10:745–758
Knowles WA, Pipkin P, Andrews N, Vyse A, Minor P, Brown DW, Miller E (2003) Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol 71:115–123
Laroni A, Giacomazzi CG, Grimaldi L, Gallo P, Sormani MP, Bertolotto A, McDermott JL, Gandoglia I, Martini I, Vitello G, Rinaldi F, Barzon L, Militello V, Pizzorno M, Bandini F, Capello E, Palù G, Uccelli A, Mancardi GL, Varnier OE (2012) Urinary JCV-DNA testing during natalizumab treatment may increase accuracy of PML risk stratification. J NeuroImmune Pharm 7:665–672
Lee P, Plavina T, Castro A, Berman M, Jaiswal D, Rivas S, Schlain B, Subramanyam M (2013) A second-generation ELISA (STRATIFY JCV DxSelect) for detection of JC virus antibodies in human serum and plasma to support progressive multifocal leukoencephalopathy risk stratification. J Clin Virol 57:141–146
Lonergan R, Carr M, De Gascun C, Costelloe LF, Waters A, Coughlan S, Duggan M, Doyle K, Jordan S, Hutchinson MW, Hall WW, Tubridy NJ (2009) Reactivation of BK polyomavirus in patients with multiple sclerosis receiving natalizumab therapy. J Neurovirol 15:351–359
Major EO (2010) Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med 61:35–47
Major EO, Frohman E, Douek D (2013a) JC viremia in natalizumab-treated patients with multiple sclerosis. N Engl J Med 368:2240–2241
Major EO, Frohman E, Douek D (2013b) More on JC viremia in natalizumab-treated patients with multiple sclerosis. N Engl J Med 369:1279–80
Mancuso R, Saresella M, Hernis A, Marventano I, Ricci C, Agostini S, Rovaris M, Caputo D, Clerici M (2012) JC virus detection and JC virus-specific immunity in natalizumab-treated multiple sclerosis patients. J Transl Med 10:248–250
Pietropaolo V, Fioriti D, Mischitelli M, Anzivino E, Santini M, Millefiorini E, Di Rezze S, Degener AM (2005) Detection of human herpesviruses and polyomaviruses DNA in a group of patients with relapsing-remitting multiple sclerosis. New Microbiol 28:199–203
Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW, Investigators AFFIRM (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354:899
Rinaldi L, Rinaldi F, Perini P, Calabrese M, Seppi D, Grossi P, Mattisi I, Barzon L, Mengoli C, Sanzari M, Palú G, Gallo P (2010) No evidence of JC virus reactivation in natalizumab treated multiple sclerosis patients: an 18 month follow-up study. J Neurol Neurosurg Psychiatry 81:1345–1350
Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW, Lublin FD, Weinstock-Guttman B, Wynn DR, Lynn F, Panzara MA, Sandrock AW, SENTINEL Investigators (2006) Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 354:911
Rudick RA, O'Connor PW, Polman CH, Rudick RA, O'Connor PW, Polman CH (2010) Assessment of JC virus DNA in blood and urine from natalizumab-treated patients. Ann Neurol 68:304–310
Sadiq SA, Puccio LM, Brydon EW (2010) JCV detection in multiple sclerosis patients treated with natalizumab. J Neurol 257:954–958
Tornatore C, Berger JR, Houff SA, Curfman B, Meyers K, Winfield D, Major EO (1992) Detection of JC virus DNA in peripheral lymphocytes from patients with and without progressive multifocal leukoencephalopathy. Ann Neurol 31:454–462
Trampe AK, Hemmelmann C, Stroet A, Haghikia A, Hellwig K, Wiendl H, Goelz S, Ziegler A, Gold R, Chan A (2012) Anti-JC virus antibodies in a large German natalizumab-treated multiple sclerosis cohort. Neurology 78:1736–1742
Tremolada S, Delbue S, Castagnoli L, Allegrini S, Miglio U, Boldorini R, Elia F, Gordon J, Ferrante P (2010) Mutations in the external loops of BK virus VP1 and urine viral load in renal transplant recipients. J Cell Phisiol 222:195–199
TYSABRI Safety Update. (2014) (Accessed January, 2014, at https://medinfo.biogenidec.com/medinfo/secure/pmlresource.do?resource=TYSABRIPMLSafetyUpdate)
Warnke C, Adams O, Hartung HP, Kieseier BC (2011) Assessment of JC virus DNA in blood and urine from natalizumab-treated patients. Ann Neurol 69:215–216
Acknowledgments
We would like to thank Dr. Federico Ambrogi from the University of Milano, Italy, for helping with the statistical analysis.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding statement
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delbue, S., Elia, F., Carloni, C. et al. JC virus urinary excretion and seroprevalence in natalizumab-treated multiple sclerosis patients. J. Neurovirol. 21, 645–652 (2015). https://doi.org/10.1007/s13365-014-0268-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-014-0268-0