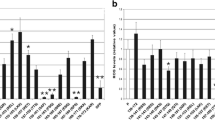
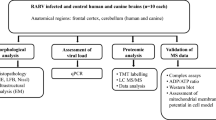
Abstract
Infection with the challenge virus standard-11 (CVS) strain of fixed rabies virus induces neuronal process degeneration in adult mice after hindlimb footpad inoculation. CVS-induced axonal swellings of primary rodent dorsal root ganglion neurons are associated with 4-hydroxy-2-nonenal protein adduct staining, indicating a critical role of oxidative stress. Mitochondrial dysfunction is the major cause of oxidative stress. We hypothesized that CVS infection induces mitochondrial dysfunction leading to oxidative stress. We investigated the effects of CVS infection on several mitochondrial parameters in different cell types. CVS infection significantly increased maximal uncoupled respiration and complex IV respiration and complex I and complex IV activities, but did not affect complex II–III or citrate synthase activities. Increases in complex I activity, but not complex IV activity, correlated with susceptibility of the cells to CVS infection. CVS infection maintained coupled respiration and rate of proton leak, indicating a tight mitochondrial coupling. Possibly as a result of enhanced complex activity and efficient coupling, a high mitochondrial membrane potential was generated. CVS infection reduced the intracellular ATP level and altered the cellular redox state as indicated by a high NADH/NAD+ ratio. The basal production of reactive oxygen species (ROS) was not affected in CVS-infected neurons. However, a higher rate of ROS generation occurred in CVS-infected neurons in the presence of mitochondrial substrates and inhibitors. We conclude that CVS infection induces mitochondrial dysfunction leading to ROS overgeneration and oxidative stress.










Similar content being viewed by others
References
Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A 105:14447–14452
Bao J, Scott I, Lu Z, Pang L, Dimond CC, Gius D, Sack MN (2010) SIRT3 is regulated by nutrient excess and modulates hepatic susceptibility to lipotoxicity. Free Radic Biol Med 49:1230–1237
Bardeletti G (1977) Respiration and ATP level in BHK21/13S cells during the earlist stages of rubella virus replication. Intervirology 8:100–109
Bernstein BW, Chen H, Boyle JA, Bamburg JR (2006) Formation of actin-ADF/cofilin rods transiently retards decline of mitochondrial potential and ATP in stressed neurons. Am J Physiol Cell Physiol 291:C828–C839
Boya P, Roumier T, Andreau K, Gonzalez-Polo RA, Zamzami N, Castedo M, Kroemer G (2003) Mitochondrion-targeted apoptosis regulators of viral origin. Biochem Biophys Res Commun 304:575–581
Boya P, Pauleau AL, Poncet D, Gonzalez-Polo RA, Zamzami N, Kroemer G (2004) Viral proteins targeting mitochondria: controlling cell death. Biochim Biophys Acta 1659:178–189
Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC (2010) Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry 49:304–311
da Silva LS, da Silva APP, Da Poian AT, El-Bacha T (2012) Mitochondrial bioenergetic alterations in mouse neuroblastoma cells infected with Sindbis virus: implications to viral replication and neuronal death. PLoS One 7:e33871
El-Bacha T, Da Poian AT (2013) Virus-induced changes in mitochondrial bioenergetics as potential targets for therapy. Int J Biochem Cell Biol 45:4110–4146
Fu ZF, Jackson AC (2005) Neuronal dysfunction and death in rabies virus infection. J Neurovirol 11:101–106
Gambini J, Gomez-Cabrera MC, Borras C, Valles SL, Lopez-Grueso R, Martinez-Bello VE, Herranz D, Pallardo FV, Tresguerres JA, Serrano M, Vina J (2011) Free [NADH]/[NAD(+)] regulates sirtuin expression. Arch Biochem Biophys 512:24–29
Gholami A, Kassis R, Real E, Delmas O, Guadagnini S, Larrous F, Obach D, Prevost MC, Jacob Y, Bourhy H (2008) Mitochondrial dysfunction in lyssavirus-induced apoptosis. J Virol 82:4774–4784
Jackson AC (ed) (2013a) Rabies: scientific basis of the disease and its management, Thirdth edn. Elsevier Academic Press, Oxford
Jackson AC (2013b) Therapy of human rabies. In: Jackson AC (ed) Rabies: scientific basis of the disease and its management, 3rd edn. Elsevier Academic Press, Oxford, pp 573–587
Jackson AC, Kammouni W, Zherebitskaya E, Fernyhough P (2010) Role of oxidative stress in rabies virus infection of adult mouse dorsal root ganglion neurons. J Virol 84:4697–4705
Kammouni W, Hasan L, Saleh A, Wood H, Fernyhough P, Jackson AC (2012) Role of nuclear factor-kB in oxidative stress associated with rabies virus infection of adult rat dorsal root ganglion neurons. J Virol 86:8139–8146
Kiebish MA, Han X, Cheng H, Chuang JH, Seyfried TN (2008) Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: lipidomic evidence supporting the Warburg theory of cancer. J Lipid Res 49:2545–2556
Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y (2006) Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell 23:607–618
Lee S, Tak E, Lee J, Rashid MA, Murphy MP, Ha J, Kim SS (2011) Mitochondrial H2O2 generated from electron transport chain complex I stimulates muscle differentiation. Cell Res 21:817–834
Li D, Wang XZ, Yu JP, Chen ZX, Huang YH, Tao QM (2004) Cytochrome C oxidase III interacts with hepatitis B virus X protein in vivo by yeast two-hybrid system. World J Gastroenterol 10:2805–2808
Li XQ, Sarmento L, Fu ZF (2005) Degeneration of neuronal processes after infection with pathogenic, but not attenuated, rabies viruses. J Virol 79:10063–10068
Li Y, Boehning DF, Qian T, Popov VL, Weinman SA (2007) Hepatitis C virus core protein increases mitochondrial ROS production by stimulation of Ca2+ uniporter activity. FASEB J 21:2474–2485
Lichty BD, McBride H, Hanson S, Bell JC (2006) Matrix protein of Vesicular stomatitis virus harbours a cryptic mitochondrial-targeting motif. J Gen Virol 87:3379–3384
Maynard ND, Gutschow MV, Birch EW, Covert MW (2010) The virus as metabolic engineer. Biotechnol J 5:686–694
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Quarato G, Scrima R, Agriesti F, Moradpour D, Capitanio N, Piccoli C (2013) Targeting mitochondria in the infection strategy of the hepatitis C virus. Int J Biochem Cell Biol 45:156–166
Roy Chowdhury SK, Zherebitskaya E, Smith DR, Akude E, Chattopadhyay S, Jolivalt CG, Calcutt NA, Fernyhough P (2010) Mitochondrial respiratory chain dysfunction in dorsal root ganglia of streptozotocin-induced diabetic rats and its correction by insulin treatment. Diabetes 59:1082–1091
Sagara J, Kawai A (1992) Identification of heat shock protein 70 in the rabies virion. Virology 190:845–848
Scott CA, Rossiter JP, Andrew RD, Jackson AC (2008) Structural abnormalities in neurons are sufficient to explain the clinical disease and fatal outcome in experimental rabies in yellow fluorescent protein-expressing transgenic mice. J Virol 82:513–521
Selivanov VA, Votyakova TV, Pivtoraiko VN, Zeak J, Sukhomlin T, Trucco M, Roca J, Cascante M (2011) Reactive oxygen species production by forward and reverse electron fluxes in the mitochondrial respiratory chain. PLoS Comput Biol 7:e1001115
Song Y, Hou J, Qiao B, Li Y, Xu Y, Duan M, Guan Z, Zhang M, Sun L (2013) Street rabies virus causes dendritic injury and F-actin depolymerization in the hippocampus. J Gen Virol 94:276–283
Stojanovski D, Johnston AJ, Streimann I, Hoogenraad NJ, Ryan MT (2003) Import of nuclear-encoded proteins into mitochondria. Exp Physiol 88:57–64
Teepker M, Anthes N, Fischer S, Krieg JC, Vedder H (2007) Effects of oxidative challenge and calcium on ATP-levels in neuronal cells. NeuroToxicology 28:19–26
Thoulouze MI, Lafage M, Schachner M, Hartmann U, Cremer H, Lafon M (1998) The neural cell adhesion molecule is a receptor for rabies virus. J Virol 72:7181–7190
Young JC, Hoogenraad NJ, Hartl FU (2003) Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell 112:41–50
Zoratti M, Szabo I (1995) The mitochondrial permeability transition. Biochim Biophys Acta 1241:139–176
Acknowledgements
This work was supported by Canadian Institutes of Health Research / Manitoba Regional Partnership Program with the Manitoba Health Research Council (to A.C. Jackson and P. Fernyhough) and the St. Boniface Hospital Research Foundation (to P. Fernyhough). We thank Dr. Ali Saleh for assistance with the ANOVA statistical analysis.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Submitted to the Journal of NeuroVirology (NJIV-D-13-00082) on August 2, 2013; Revised and resubmitted on September 20, 2013
Rights and permissions
About this article
Cite this article
Alandijany, T., Kammouni, W., Roy Chowdhury, S.K. et al. Mitochondrial dysfunction in rabies virus infection of neurons. J. Neurovirol. 19, 537–549 (2013). https://doi.org/10.1007/s13365-013-0214-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-013-0214-6