Abstract
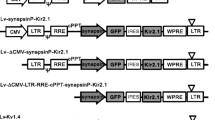
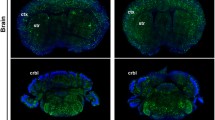
We previously reported that vesicular stomatitis virus-derived glycoprotein (VSV-G)-pseudotyped lentiviral vectors harvested 2 days post-transfection preferred to infect Purkinje cells (PCs), whereas those harvested after a longer cultivation period exhibited Bergmann glia-preferential transduction. However, the mechanisms by which lentiviral tropism was altered remained unsolved. Here, we investigated whether proteases released from the cells during viral production affect lentiviral tropism. Enhanced green fluorescence protein-expressing lentiviral vectors were produced using human embryonic kidney (HEK) 293FT or 293 T cells and injected into the mouse cerebellum to examine tropism in PCs. We found that the addition of a protease inhibitor—in particular, the cathepsin K (CatK) inhibitor—into the culture medium significantly increased lentiviral tropism in PCs. Moreover, the concentration of CatK in the culture medium drastically increased upon prolonged cultivation, concomitant with the expression levels of CatK in HEK 293 T cells. An increase in CatK activity by the addition of recombinant CatK enzyme to PC-preferential viral solution, which was obtained 2 days post-transfection, shifted the viral tropism toward Bergmann glia. In contrast, a decrease in CatK activity in the Bergmann glia-preferential viral solution, which was obtained 6 days post-transfection by the addition of CatK inhibitor or by the removal of a CatK-containing fraction, restored the PC preference of viruses. These results suggest that the CatK released from deteriorated HEK 293 T cells plays a key role in reducing lentiviral tropism in PCs, presumably by affecting a receptor molecule for lentiviral VSV-G, resulting in the preferential transduction of Bergmann glia.






Similar content being viewed by others
References
Bernstein HG, Bukowska A, Dobrowolny H, Bogerts B, Lendeckel U (2007) Cathepsin K and schizophrenia. Synapse 61:252–253
Bossard MJ, Tomaszek TA, Thompson SK, Amegadzie BY, Hanning CR, Jones C, Kurdyla JT, McNulty DE, Drake FH, Gowen M, Levy MA (1996) Proteolytic activity of human osteoclast cathepsin K. Expression, purification, activation, and substrate identification. J Biol Chem 271:12517–12524
Drake FH, Dodds RA, James IE, Connor JR, Debouck C, Richardson S, Lee-Rykaczewski E, Coleman L, Rieman D, Barthlow R, Hastings G, Gowen M (1996) Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J Biol Chem 271:12511–12516
Garnero P, Borel O, Byrjalsen I, Ferreras M, Drake FH, McQueney MS, Foged NT, Delmas PD, Delaisse JM (1998) The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem 273:32347–32352
Graham FL, Smiley J, Russell WC, Nairn R (1977) Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 36:59–74
Niwa H, Yamamura K, Miyazaki J (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199
Quintanilla-Dieck MJ, Codriansky K, Keady M, Bhawan J, Runger TM (2009) Expression and regulation of cathepsin K in skin fibroblasts. Exp Dermatol 18:596–602
Runger TM, Quintanilla-Dieck MJ, Bhawan J (2007) Role of cathepsin K in the turnover of the dermal extracellular matrix during scar formation. J Invest Dermatol 127:293–297
Shaw G, Morse S, Ararat M, Graham FL (2002) Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J 16:869–871
Torashima T, Okoyama S, Nishizaki T, Hirai H (2006a) In vivo transduction of murine cerebellar Purkinje cells by HIV-derived lentiviral vectors. Brain Res 1082:11–22
Torashima T, Yamada N, Itoh M, Yamamoto A, Hirai H (2006b) Exposure of lentiviral vectors to subneutral pH shifts the tropism from Purkinje cell to Bergmann glia. Eur J Neurosci 24:371–380
Vaillant C, Didier-Bazes M, Hutter A, Belin MF, Thomasset N (1999) Spatiotemporal expression patterns of metalloproteinases and their inhibitors in the postnatal developing rat cerebellum. J Neurosci 19:4994–5004
Yee JK, Friedmann T, Burns JC (1994) Generation of high-titer pseudotyped retroviral vectors with very broad host range. Methods Cell Biol 43 Pt A:99–112
Acknowledgments
This work was supported in part by KAKENHI (19670003), the Funding Program for Next Generation World-Leading Researchers (LS021), and grants from Research on Measures for Intractable Diseases (Ataxic Diseases and Neurodegenerative Diseases) from the Ministry of Health, Labour and Welfare (to H. Hirai). The lentiviral vector and MSCV promoter were provided by St. Jude Children’s Research Hospital and the American National Red Cross, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goenawan, H., Hirai, H. Modulation of lentiviral vector tropism in cerebellar Purkinje cells in vivo by a lysosomal cysteine protease cathepsin K. J. Neurovirol. 18, 521–531 (2012). https://doi.org/10.1007/s13365-012-0134-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-012-0134-x