Abstract
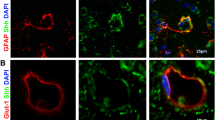
Understanding the mechanisms of neuronal regeneration and repair in the adult central nervous system is a vital area of research. Using a rhesus lentiviral encephalitis model, we sought to determine whether recovery of neuronal metabolism after injury coincides with the induction of two important markers of synaptodendritic repair: growth-associated protein-43 (GAP-43) and ephrin B3. We examined whether the improvement of neuronal metabolism with combined anti-retroviral therapy (cART) after simian immunodeficiency virus (SIV) infection in rhesus macaques involved induction of GAP-43, also known as neuromodulin, and ephrin B3, both implicated in axonal pathfinding during neurodevelopment and regulation of synapse formation, neuronal plasticity, and repair in adult brain. We utilized magnetic resonance spectroscopy to demonstrate improved neuronal metabolism in vivo in adult SIV-infected cART animals compared to untreated and uninfected controls. We then assessed levels of GAP-43, ephrin B3, and synaptophysin, a pre-synaptic marker, in three brain regions important for cognitive function, cortex, hippocampus, and putamen, by quantitative real-time RT-PCR and immunohistochemistry. Here we demonstrate that (1) GAP-43 mRNA and protein are induced with SIV infection, (2) GAP-43 protein is higher in the hippocampus outer molecular layer in SIV-infected animals that received cART compared to those that did not, and (3) activated microglia and infiltrating SIV-infected macrophages express abundant ephrin B3, an important axonal guidance molecule. We propose a model whereby SIV infection triggers events that lead to induction of GAP-43 and ephrin B3, and that short-term cART results in increased magnitude of repair mechanisms especially in the hippocampus, a region known for high levels of adult plasticity.







Similar content being viewed by others
References
Alirezaei M, Watry DD, Flynn CF, Kiosses WB, Masliah E, Williams BR, Kaul M, Lipton SA, Fox HS (2007) Human immunodeficiency virus-1/surface glycoprotein 120 induces apoptosis through RNA-activated protein kinase signaling in neurons. J Neurosci 27:11047–11055
Annamalai L, Bhaskar V, Pauley DR, Knight H, Williams K, Lentz M, Ratai E, Westmoreland SV, Gonzalez RG, O'Neil SP (2010) Impact of short-term combined antiretroviral therapy on brain virus burden in simian immunodeficiency virus-infected and CD8+ lymphocyte-depleted rhesus macaques. Am J Pathol 177:777–791
Benowitz LI, Routtenberg A (1997) GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci 20:84–91
Benowitz LI, Apostolides PJ, Perrone-Bizzozero N, Finklestein SP, Zwiers H (1988) Anatomical distribution of the growth-associated protein GAP-43/B-50 in the adult rat brain. J Neurosci 8:339–352
Benowitz LI, Perrone-Bizzozero NI, Finklestein SP, Bird ED (1989) Localization of the growth-associated phosphoprotein GAP-43 (B-50, F1) in the human cerebral cortex. J Neurosci 9:990–995
Benowitz LI, Perrone-Bizzozero NI, Neve RL, Rodriguez W (1990a) GAP-43 as a marker for structural plasticity in the mature CNS. Prog Brain Res 86:309–320
Benowitz LI, Rodriguez WR, Neve RL (1990b) The pattern of GAP-43 immunostaining changes in the rat hippocampal formation during reactive synaptogenesis. Brain Res Mol Brain Res 8:17–23
Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF (2005) Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci U S A 102:10694–10699
Bissel SJ, Wang G, Ghosh M, Reinhart TA, Capuano S 3rd, Stefano Cole K, Murphey-Corb M, Piatak M Jr, Lifson JD, Wiley CA (2002) Macrophages relate presynaptic and postsynaptic damage in simian immunodeficiency virus encephalitis. Am J Pathol 160:927–941
Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S (2005) Growth-associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol 193:291–311
Carmona MA, Murai KK, Wang L, Roberts AJ, Pasquale EB (2009) Glial ephrin-A3 regulates hippocampal dendritic spine morphology and glutamate transport. Proc Natl Acad Sci U S A 106:12524–12529
Chumley MJ, Catchpole T, Silvany RE, Kernie SG, Henkemeyer M (2007) EphB receptors regulate stem/progenitor cell proliferation, migration, and polarity during hippocampal neurogenesis. J Neurosci 27:13481–13490
Everall IP, Trillo-Pazos G, Bell C, Mallory M, Sanders V, Masliah E (2001) Amelioration of neurotoxic effects of HIV envelope protein gp120 by fibroblast growth factor: a strategy for neuroprotection. J Neuropathol Exp Neurol 60:293–301
Everall IP, Bell C, Mallory M, Langford D, Adame A, Rockestein E, Masliah E (2002) Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol Cell Neurosci 21:493–501
Filosa A, Paixao S, Honsek SD, Carmona MA, Becker L, Feddersen B, Gaitanos L, Rudhard Y, Schoepfer R, Klopstock T, Kullander K, Rose CR, Pasquale EB, Klein R (2009) Neuron-glia communication via EphA4/ephrin-A3 modulates LTP through glial glutamate transport. Nat Neurosci 12:1285–1292
Fox HS, Weed MR, Huitron-Resendiz S, Baig J, Horn TF, Dailey PJ, Bischofberger N, Henriksen SJ (2000) Antiviral treatment normalizes neurophysiological but not movement abnormalities in simian immunodeficiency virus-infected monkeys. J Clin Invest 106:37–45
Furne C, Ricard J, Cabrera JR, Pays L, Bethea JR, Mehlen P, Liebl DJ (2009) EphrinB3 is an anti-apoptotic ligand that inhibits the dependence receptor functions of EphA4 receptors during adult neurogenesis. Biochim Biophys Acta 1793:231–238
Gonzalez RG, Cheng LL, Westmoreland SV, Sakaie KE, Becerra LR, Lee PL, Masliah E, Lackner AA (2000) Early brain injury in the SIV-macaque model of AIDS. AIDS 14:2841–2849
Greco JB, Westmoreland SV, Ratai EM, Lentz MR, Sakaie K, He J, Sehgal PK, Masliah E, Lackner AA, Gonzalez RG (2004) In vivo 1H MRS of brain injury and repair during acute SIV infection in the macaque model of neuroAIDS. Magn Reson Med 51:1108–1114
Gupta RG, Kelly KM, Helke KL, Queen SE, Karper JM, Dorsey JL, Brice AK, Adams RJ, Tarwater PM, Kolson DL, Mankowski JL (2010) HIV and SIV induce alterations in CNS CaMKII expression and activation: a potential mechanism for cognitive impairment. Am J Pathol 176:2776–2784
Haworth SJ, Christofalo B, Anderson RD, Dunkle LM (1998) A single-dose study to assess the penetration of stavudine into human cerebrospinal fluid in adults. J Acquir Immune Defic Syndr Hum Retrovirol 17:235–238
Higo N, Nishimura Y, Murata Y, Oishi T, Yoshino-Saito K, Takahashi M, Tsuboi F, Isa T (2009) Increased expression of the growth-associated protein 43 gene in the sensorimotor cortex of the macaque monkey after lesioning the lateral corticospinal tract. J Comp Neurol 516:493–506
Irwin N, Chao S, Goritchenko L, Horiuchi A, Greengard P, Nairn AC, Benowitz LI (2002) Nerve growth factor controls GAP-43 mRNA stability via the phosphoprotein ARPP-19. Proc Natl Acad Sci U S A 99:12427–12431
Irwin N, Li YM, O'Toole JE, Benowitz LI (2006) Mst3b, a purine-sensitive Ste20-like protein kinase, regulates axon outgrowth. Proc Natl Acad Sci U S A 103:18320–18325
Jacobson RD, Virag I, Skene JH (1986) A protein associated with axon growth, GAP-43, is widely distributed and developmentally regulated in rat CNS. J Neurosci 6:1843–1855
Klein R (2009) Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci 12:15–20
Lentz MR, Kim JP, Westmoreland SV, Greco JB, Fuller RA, Ratai EM, He J, Sehgal PK, Halpern EF, Lackner AA, Masliah E, Gonzalez RG (2005) Quantitative neuropathologic correlates of changes in ratio of N-acetylaspartate to creatine in macaque brain. Radiology 235:461–468
Lentz MR, Westmoreland SV, Lee V, Ratai EM, Halpern EF, Gonzalez RG (2008) Metabolic markers of neuronal injury correlate with SIV CNS disease severity and inoculum in the macaque model of neuroAIDS. Magn Reson Med 59:475–484
Lipton SA, Brenneman DE, Silverstein FS, Masliah E, Mucke L (1995) gp120 and neurotoxicity in vivo. Trends Pharmacol Sci 16:122
Liu X, Hawkes E, Ishimaru T, Tran T, Sretavan DW (2006) EphB3: an endogenous mediator of adult axonal plasticity and regrowth after CNS injury. J Neurosci 26:3087–3101
Masliah E, Achim CL, Ge N, DeTeresa R, Terry RD, Wiley CA (1992a) Spectrum of human immunodeficiency virus-associated neocortical damage. Ann Neurol 32:321–329
Masliah E, Ge N, Achim CL, Hansen LA, Wiley CA (1992b) Selective neuronal vulnerability in HIV encephalitis. J Neuropathol Exp Neurol 51:585–593
Masliah E, Ge N, Morey M, DeTeresa R, Terry RD, Wiley CA (1992c) Cortical dendritic pathology in human immunodeficiency virus encephalitis. Lab Invest 66:285–291
Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I (1997) Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol 42:963–972
Neve RL, Finch EA, Bird ED, Benowitz LI (1988) Growth-associated protein GAP-43 is expressed selectively in associative regions of the adult human brain. Proc Natl Acad Sci U S A 85:3638–3642
Perrone-Bizzozero NI, Weiner D, Hauser G, Benowitz LI (1988) Extraction of major acidic Ca2+ dependent phosphoproteins from synaptic membranes. J Neurosci Res 20:346–350
Ratai EM, Bombardier JP, Joo CG, Annamalai L, Burdo TH, Campbell J, Fell R, Hakimelahi R, He J, Autissier P, Lentz MR, Halpern EF, Masliah E, Williams KC, Westmoreland SV, Gonzalez RG (2010) Proton magnetic resonance spectroscopy reveals neuroprotection by oral minocycline in a nonhuman primate model of accelerated NeuroAIDS. PLoS One 5:e10523
Ratai EM, Pilkenton S, He J, Fell R, Bombardier JP, Joo CG, Lentz MR, Kim WK, Burdo TH, Autissier P, Annamalai L, Curran E, O'Neil SP, Westmoreland SV, Williams KC, Masliah E, Gilberto Gonzalez R (2011) CD8(+) lymphocyte depletion without SIV infection does not produce metabolic changes or pathological abnormalities in the rhesus macaque brain. J Med Primatol. doi:10.1111/j.1600-0684.2011.00475.x
Schinazi RF, Boudinot FD, Ibrahim SS, Manning C, McClure HM, Liotta DC (1992) Pharmacokinetics and metabolism of racemic 2′,3′-dideoxy-5-fluoro-3′-thiacytidine in rhesus monkeys. Antimicrob Agents Chemother 36:2432–2438
Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA (1999) Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857–860
Strittmatter SM, Vartanian T, Fishman MC (1992) GAP-43 as a plasticity protein in neuronal form and repair. J Neurobiol 23:507–520
Westmoreland SV, Kolson D, Gonzalez-Scarano F (1996) Toxicity of TNF alpha and platelet activating factor for human NT2N neurons: a tissue culture model for human immunodeficiency virus dementia. J Neurovirol 2:118–126
Westmoreland SV, Alvarez X, deBakker C, Aye P, Wilson ML, Williams KC, Lackner AA (2002) Developmental expression patterns of CCR5 and CXCR4 in the rhesus macaque brain. J Neuroimmunol 122:146–158
Wiley CA, Masliah E, Morey M, Lemere C, DeTeresa R, Grafe M, Hansen L, Terry R (1991) Neocortical damage during HIV infection. Ann Neurol 29:651–657
Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG (2005) Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest 115:2534–2545
Xie Z, Westmoreland SV, Bahn ME, Chen GL, Yang H, Vallender EJ, Yao WD, Madras BK, Miller GM (2007) Rhesus monkey trace amine-associated receptor 1 signaling: enhancement by monoamine transporters and attenuation by the D2 autoreceptor in vitro. J Pharmacol Exp Ther 321:116–127
Xu NJ, Henkemeyer M (2009) Ephrin-B3 reverse signaling through Grb4 and cytoskeletal regulators mediates axon pruning. Nat Neurosci 12:268–276
Yu G, Luo H, Wu Y, Wu J (2003) Mouse ephrinB3 augments T-cell signaling and responses to T-cell receptor ligation. J Biol Chem 278:47209–47216
Acknowledgments
We would like to thank Dr. Larry Benowitz for supplying the anti- GAP-43 antibody and protocol, Ron Desrosiers for supplying the virus inoculum SIVmac251, Keith Reimann for providing anti-CD8 cM-T807, Raymond Schinazi for supplying Racivir (RCV), and Norbert Bischofberger of Gilead Sciences for providing PMPA. In addition, we thank Elizabeth Curran, Michael O’Connell, and Doug Pauley for pathology assistance, and Drs. Prabhat Sehgal, Angela Carville, and Elisabeth Moeller for veterinary expertise. We also thank Hayley Dirscherl, Alexis Denysyk, Heather Knight, and Karen Boisvert Dalecki for their contribution with immunohistochemistry and Hong Yang for real-time PCR contribution. This work was supported by RR00168 (NEPRC Base Grant), R01NS050041 (RGG), DA025697 (GMM), and the NEPRC Microscopy and Primate Genetics Cores.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Westmoreland, S.V., Annamalai, L., Lentz, M.R. et al. Growth-associated protein-43 and ephrin B3 induction in the brain of adult SIV-infected rhesus macaques. J. Neurovirol. 17, 455–468 (2011). https://doi.org/10.1007/s13365-011-0047-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-011-0047-0