Abstract
Concern has been raised regarding red fox (Vulpes Vulpes) population increase and range expansion into alpine tundra, directly and indirectly enhanced by human activities, including carrion supply, and its negative impact on native fauna. In this study, we used cameras on bait stations and hunting remains to investigate how spatiotemporal patterns of red fox scavenging were influenced by abundance and accessibility of live prey, i.e., small rodent population cycles, snow depth, and primary productivity. We found contrasting patterns of scavenging between habitats during winter. In alpine areas, use of baits was highest post rodent peaks and when snow depth was low. This probably reflected relatively higher red fox abundance due to increased reproduction or migration of individuals from neighboring areas, possibly also enhanced by a diet shift. Contrastingly, red fox use of baits in the forest was highest during rodent low phase, and when snow was deep, indicating a higher dependency of carrion under these conditions. Scavenging patterns by red fox on the pulsed but predictable food resource from hunting remains in the autumn revealed no patterns throughout the rodent cycle. In this study, we showed that small rodent dynamics influenced red fox scavenging, at least in winter, but with contrasting patterns depending on environmental conditions. In marginal alpine areas, a numerical response to higher availability of rodents possible lead to the increase in bait visitation the proceeding winter, while in more productive forest areas, low availability of rodents induced a functional diet shift towards scavenging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecosystems are subsidized to a varying degree with energy, matter, and organisms from neighboring sources (Polis et al. 1997; Leroux and Loreau 2008), or by anthropogenic activity (Oro et al. 2013). Such subsidies can cause an increase in abundance and distribution of opportunistic species which, in turn, may result in trophic cascades altering communities, or ecological processes such as competition and predator-prey interactions (Tylianakis et al. 2008; Oro et al. 2013; Rød-Eriksen et al. 2020). Generalist predators may depend on resource subsidies in periods when their primary prey species are less abundant, less vulnerable, or unavailable (Pereira et al. 2014). Subsidies can therefore alter predator population dynamics, with consecutive effects on prey populations (Newsome et al. 2015).
The red fox is a generalist predator occupying a wide range of ecosystems around the globe (Schipper et al. 2008), and it is listed among the 100 most invasive species outside its original range (Lowe et al. 2000). Increasing and expanding populations of red fox raise concern, as they may negatively affect both populations of endangered species and important game species (Fletcher et al. 2010; Jahren et al. 2016; Elmhagen et al. 2017; Marolla et al. 2019; Rød-Eriksen et al. 2020). In Fennoscandia, both direct and indirect factors connected to anthropogenic activity have been linked to red fox range expansion and increased abundance. For example, top-down regulation of red foxes has probably been reduced, both due to intense lethal control of large carnivores and reduced hunting pressure from humans (Selås and Vik 2006; Pasanen-Mortensen et al. 2013). Additionally, land use changes have increased areas of crop production and clear-cuts in the forests which, in turn, benefits small rodents and roe deer (Capreolus capreolus), which are important food resources for the red fox (Pasanen-Mortensen et al. 2017). Moreover, red foxes might benefit from increased availability of anthropogenic food resources through the expansion of human settlements, cabin areas, and infrastructure (Gallant et al. 2020; Rød-Eriksen et al. 2020). Species of facultative scavengers in northern ecosystems often utilize carrion during winter, creating possibilities for food web interactions between species that otherwise have weak connections in these ecosystems (Ims and Fuglei 2005). Red foxes are facultative scavengers, and ungulate carrion is often an important part of their diet, especially during winter (Jędrzejewski and Jędrzejewska 1992; Killengreen et al. 2011; Needham et al. 2014) when the availability of prey is lower (Cagnacci et al. 2003). Hence, increasing densities of ungulate populations, and thereby carrion, have been linked to increased winter survival and elevated carrying capacity of red foxes in Fennoscandia during the last century (Selås and Vik 2006). More recent data from the arctic tundra in Scandinavia also links higher abundance of carrion from semi-domesticated reindeer (Rangifer tarandus) to higher occupancy and range expansion of scavenging species including red fox, corvids, and eagles (Henden et al. 2014; Sokolov et al. 2016). Remains from hunting of ungulates are another source of food that can subsidize scavengers during harvest periods (Wikenros et al. 2013; Gomo et al. 2017).
Elevated carrying capacities due to climate change are expected for some boreal generalist predator species in northern ecosystems (Elmhagen et al. 2015). Since climate change affects a wide range of species and ecological processes, the overall outcome is likely to be diverse and vary among regions. For example, small rodent cyclicity is an important component of northern ecosystems, where many mammalian and avian predators are adapted to respond quickly to high small rodent abundances (Ims and Fuglei 2005; Gilg et al. 2012). Since the reproductive success of many of these species is closely dependent on small rodent abundance, dampening of multiannual small rodent population cycles due to climate change has been a matter of concern during the last decades (Kausrud et al. 2008; Henden et al. 2009; Schmidt et al. 2012; Ehrich et al. 2020). Elevated winter temperatures alter snow conditions and may lead to a collapse of subnivean space, which in turn entails higher winter mortality in small rodents (Kausrud et al. 2008). Changes in snow conditions might also affect the availability of small rodents for predators (Jędrzejewski and Jędrzejewska 1992; Lindström and Hörnfeldt 1994). Climate-driven changes in small rodent abundance and availability may thus affect red fox survival and carrying capacity, and possibly affect the importance of carrion and anthropogenic food resources in their diet.
The aim of this paper was to investigate how spatiotemporal patterns of red fox scavenging are influenced by factors affecting abundance and accessibility of live prey. Based on camera trap data, we explored how temporal variation in main prey abundance, i.e., small rodents, and other environmental factors affected red fox visitation rates at bait stations and hunting remains. By using bait stations both in forest and alpine tundra areas in winter, as well as hunting remains from moose (Alces alces) in autumn, the study covered contrasting environmental conditions regarding productivity, small rodent abundance, carrion availability, and snow depth (Hagen 2014; Gomo et al. 2017).
We formulated two hypotheses, that were not mutually exclusive, and investigated their validity by evaluating a set of predictions: According to the (i) numerical response hypothesis (NRH), visitation rates at baits and hunting remains should mainly reflect spatiotemporal variation in red fox densities. Small rodent fluctuations have been linked to red fox reproduction (Englund 1970), and population dynamics of foxes may be positively related to small rodent abundance through a numerical response (Englund 1980). Hence, based on the NRH, we predicted that red fox visitation rates at carrion should be higher following summers of high small rodent abundance if scavenging patterns mainly reflect red fox abundance. Moreover, we predicted that visitation rates should be higher in forests than in alpine areas, and positively correlated to net primary productivity (EVI), due to overall higher and more diverse prey availability. We also predicted an inverse relationship between visitation rates and snow depth because deep snow limits the accessibility to small rodents during winter (Willebrand, Willebrand et al. 2017). However, some studies have shown that scavenging by red foxes increases in areas and in periods when prey availability is low due to diet shifts (i.e., a functional response, (Jędrzejewski and Jędrzejewska 1992, Killengreen et al. 2011)). Hence, according to the (ii) functional response hypothesis (FRH), visitation rates at gut piles and baits should be negatively correlated with live prey abundance and accessibility. FRH thus predicts that visitation rates should be higher in alpine areas where prey species’ diversity and densities are generally lower. Lastly, we predicted that scavenging should increase following the low phase of the small rodent cycle, and in areas with a low net primary productivity and in periods when deep snow limits the accessibility to small rodents.
Study area
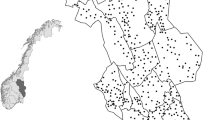
Our study was conducted in central Norway from 2012 to 2014 (Fig. 1). The bait study took place in winter between January and April, and the gut piles study (Gomo et al. 2017) in autumn between 25th September to 14th December, i.e., during and after the moose hunting season. The area covers an elevational gradient ranging from 90 to 850 m.a.s.l., with the forest line at approximately 560 m.a.s.l. (Fig. 1). Alpine tundra habitats are dominated by dwarf birch (Betula nana) and shrubs of willow (Salix sp.), whereas forested habitats are dominated by pine (Pinus sylvestris), spruce (Picea abies), and mountain birch (Betula pubescens) (Moen 1998). Semi-domesticated reindeer have perennial pastures in the region, including calving areas within or bordering our study area. Wild ungulates are mainly moose and roe deer. Carrion from ungulates provides an estimated biomass of 29.1 kg/km2 in boreal forest and 3.6 kg/km2 in alpine areas during the cold season (November to April) (Hagen 2014). Estimated biomass of moose gut piles in the gut pile study area was 33 kg/km2 (25th September to 14th November). For details, see Gomo et al. (2017).
Spatial distribution of bait study sites in central Norway. Circles show the placement of camera traps in boreal habitats (orange) and alpine tundra habitats (blue) in winter. Gray areas represent alpine tundra habitat. The red square shows the area of the gut pile study in autumn (details in Gomo et al. (2017)), whereas red stars represent small rodent trapping locations
Field sampling
Camera traps (Reconyx Hyperfire Professional PC 800 and PC 900, Reconyx Inc., WI, USA) were set up at 38 bait locations in winter (29 in forest and 9 in alpine tundra habitat) for a total of 65 bait sessions (42 in forest and 23 in alpine tundra habitat), i.e., 13 bait locations were reused several years. This resulted in a total of 1253 monitoring days between January and April, 2012–2014. The average duration of a bait session was 19 ± 4 days (mean ± 2SE) in forested and 20 ± 5 days in alpine tundra habitats, ranging from 4 to 62 days. Bait consisted of frozen blocks of discarded reindeer meat, fat, and connective tissue weighing between 10 and 20 kg and measuring approximately 60 × 40 × 15 cm. To ensure that the bait was not removed immediately by large scavengers, and to keep the bait frozen, the bait was buried in the snow in a vertical position (only 5 cm of the top was visible). In the autumn, camera traps (Reconyx Hyperfire PC 900 and Wingcam II TL) were mounted at hunting remains from 50 shot moose during the moose hunting seasons of 2012–2014, totaling 1043 monitoring days. The hunter’s field dress the moose at the site of the kill and leave the stomach, intestines, and most internal organs at the site (i.e., gut piles) while the rest is brought back for slaughter. Before leaving the dressing site, hunters set up a camera at the site facing the gut pile (Gomo et al. 2017).
Cameras were placed 4–6 m from the baits/gut piles at approximately 1−1.5 m above the ground and tilted slightly downwards towards the bait. Camera traps in alpine areas were programmed to take a picture every 5 min, whereas cameras in the forest were programmed to take a picture every 10 min. The difference in time intervals was due to varying study designs, where the camera traps in alpine tundra were part of the projects “Felles Fjellrev” I and II (see “Funding”). However, both camera settings ensured a high capture probability of elusive species (Hamel et al. 2013). To avoid differences in detection probability between forest and alpine baits, every other picture was removed from the alpine data before statistical analyses. The cameras at gut piles were in addition triggered by a motion sensor, with a 2-min delay between triggers to maintain battery and memory card capacity. All pictures were examined, and the species observed in each picture was recorded. To reduce sampling bias, only images where the bait was still present (i.e., not fully consumed), and thus acting as an attractant to animals, were included in the analyses. Complete consumption was estimated based on visual examination of the pictures.
Small rodent phases
Small rodent phases were categorized based on autumn snap trapping in two locations within the study area (Fig. 1) (Sørensen 2019; Sørensen 2020). The easternmost location (Fig. 2) was in the sub-alpine coniferous forest at a higher altitude than the more boreal westernmost location (Fig. 2). The two snap-trapping indexes showed similar cyclicity; however, the amplitudes at the eastern location were more pronounced than at the western location, likely representing a more alpine characteristic cyclicity pattern at higher altitudes (Andreassen et al. 2020). Bank vole (Myodes glareolus) was the dominating species, and Norwegian lemming (Lemmus lemmus) was caught only in the eastern location in 2011 during a pronounced lemming peak in alpine areas. Based on the abundance indices from the snap trapping, the rodent phase peaked in autumn of 2011, dipped to a low in 2012, followed by a pre-peak in 2013, and new peak in 2014. The small rodent phases used for the winter bait analyses were defined based on the abundance indexes in the preceding autumn following Stoessel et al. (2019). Hence, there was a post-peak phase during the winter of 2011–2012, following the peak in autumn 2011. Similar, rodent abundance was considered still at a low phase during winter 2012–2013, after low abundance in autumn 2012, and in a pre-peak phase in winter of 2013–2014, following an increasing abundance in autumn 2013.
Autumn snap-trapping indices for small rodents in boreal forest (the westernmost trapping location; green line) and in sub-alpine conifer forest (the easternmost trapping location; blue line) habitat. During our winter study period, a post-peak rodent phase occurred in winter 2011/12 (star), a low rodent phase in winter 2012/13 (down arrow), and a pre-peak rodent phase in winter 2013/14 (up arrow)
Environmental variables
Snow depth was extracted from interpolated maps (NMI 2019) with a resolution of 1 km pixels, and calculated as an average within a 1.5 km buffer (~7 km2) around each camera site for each bait session. Mean site snow depth (± 2SE) was 32 cm (± 9.9) at forested and 108 cm (± 9.9) at alpine sites but varied between years (Fig. 3). For primary productivity, we used a measure of peak plant productivity (average Enhanced Vegetation Index EVI), averaged over the years 2000–2018, at a resolution of 210 m pixels (for details, see Tveraa et al. 2013).
Statistical analyses
To investigate the influence of small rodent phases, snow depth, or primary productivity on the probability of daily use of carrion by red foxes, we used binomial generalized linear mixed-effects models (GLMM; in R-package lme4 (Bates et al. 2015)). As the dependent variable, we used daily presence of red fox to gut piles/baits (0 or 1, where 1 is defined as ≥1 red fox picture). We included gut pile/bait ID (N = 50 for gut piles, N = 23 for alpine baits, N = 42 for forest baits, Fig. 1) as random intercept in the models to account for repeated measures within and between years. We analyzed gut piles, forest baits, and alpine baits separately, as the sample size was too small to support three-way interactions. We included productivity, small rodent population phase, snow depth, and an interaction between small rodent phase and snow depth as explanatory variables in the bait models. The gut pile models included only productivity and small rodent phase as explanatory variables as snow cover was limited to a few days during the autumn study period. In addition, we included an estimate of local gut pile density, as this parameter was shown to affect mammal scavenging in this study area (for more details, see Gomo et al. 2017).
To compare the probability of daily use of carrion by red foxes between the three sources (gut piles, forest baits, alpine baits), we ran an additional GLMM model, including gut pile/bait ID as a random intercept.
Results
The probability of daily use of carrion by red fox was in general higher at alpine baits (predicted probabilities: 0.24 ± 0.04 SE) than at forest baits (0.12 ± 0.02 SE) in winter and on gut piles in autumn (0.10 ± 0.01 SE).
The probability of daily use by foxes at baits in alpine tundra was primarily influenced by a combination of small rodent phases and snow depth (Tables 1 and 2). Use was highest during the post-peak small rodent phase but decreased with increasing snow depth. The same pattern was observed also during the pre-peak phases, while snow depth had no effect during the low small rodent phase (Fig. 4a, Table 2). The second-best model additionally included productivity, but the increase in AICC with almost 2 (1.85) and the significantly lower AICC -weight (0.55 vs 0.22) indicate that this variable had little effect on daily use. Furthermore, the probability of daily use by foxes at baits in forest habitat was best explained by small rodent phase alone (Table 1). Use was lower in the pre-peak rodent phase compared to the post-peak (β = − 1.3, SE = 0.31, p < 0.001), but not different from the low phases (β = 0.042, SE = 0.35, p = 0.9). However, the model including an interaction with snow depth performed almost equally well (Δ AICC = 0.18; Table 1). In contrast to alpine baits, red fox daily use of forest baits increased with increasing snow depth during the low small rodent phase, while snow had little effect in the two other small rodent phases (Fig. 4b, Table 2).
Predicted probabilities of red fox daily use of bait stations in forest (a) and alpine (b) habitats during winter. The predictions are based on the model including the interaction between small rodent phases (low, pre-peak, post-peak) and snow depth (top-ranked model for alpine and second-ranked model for forest following AICC; Table 1). Dots are daily non-detections (bottom) and detections (top) from the raw data, color-coded for each rodent phase
None of the models of the probability of daily use by red fox at gut piles during the autumn performed well and the best model was only 1.29 AICC -units from the NULL model (Table S1).
The best model included only the estimated density of gut piles where red fox use was highest at intermediate densities of gut piles. The second-best model (Δ AICC = 0.25) additionally included small rodent phase which showed a tendency for higher use of gut piles during the pre-peak small rodent phase (predicted probability: 0.14 ± 0.036 SE) compared to the low phase (0.06 ± 0.028). Use of gut piles during the peak year was at intermediate levels (0.08 ± 0.031).
Discussion
This study presents novel insight into spatial and temporal patterns of red fox scavenging and its relationship with small rodent dynamics, snow depth, and habitat in central Fennoscandia. We addressed two hypotheses regarding red fox scavenging that were based on differences in the relative importance of numerical response hypothesis (NRH) and functional response hypothesis (FRH) to variation in prey abundance and accessibility. Interestingly, we found evidence supporting both hypotheses, as we detected contrasting patterns of red fox scavenging comparing productive forest and marginal alpine tundra habitats.
According to the NRH, we predicted that scavenging should increase following rodent population peaks, as red foxes have been shown to respond numerically to small rodents by increased reproduction in peak years in northern areas (Englund 1980). In our study, scavenging at baits in alpine tundra habitat was highest in the winter after a rodent peak. Increasing activity of red fox in alpine tundra habitats in winters after rodent peaks has also been found by Stoessel et al. (2019) based on snow-track counts. Following NRH, increased activity at baits may reflect an increased red fox abundance due to increased reproduction from the previous summer, or migration of individuals from neighboring areas, seeking areas with high prey availability. Concurrently, the crash phase following a rodent peak year could occur during the proceeding winter and spring, depending on winter severity (Kausrud et al. 2008), facilitating a numerical response to a continued high rodent abundance. Following FRH, high activity at baits during these conditions could also reflect diet shifts towards carcasses if the rodent population crashed in autumn or early winter. Killengreen et al. (2011) found a marked shift in the diet of red foxes living in Arctic tundra habitats, tracking the availability of small rodents, and shifting to reindeer carcasses when the availability of rodents was low. Likely, we find a combination of both these responses in alpine tundra habitats.
In contrast to alpine baits, utilization of baits in forest by red foxes peaked in the winter after a rodent low phase. This could imply a lower numerical response to small rodents in forested habitats compared to alpine tundra habitats. Small rodent abundance probably fluctuates with lower amplitude within the forest habitat of our study area (Fig. 2), where carrion biomass is also much higher (Hagen 2014). Both these factors might, in addition to higher diversity and stability of other food sources, lead to more stable red fox abundance in forest habitats (Jahren et al. 2020). Concurrently, increased activity at baits during winters with low rodent abundance may indicate a diet shift, as predicted by the FRH.
At alpine baits, red fox presence decreased with increasing snow depth in the post-peak and pre-peak small rodent phases. Red fox space use has previously been shown to be influenced by snow conditions (Pozzanghera et al. 2016), and by an interaction between snow conditions and abundance of prey and carrion (Carricondo-Sanchez et al. 2016). Even if red foxes are capable of locating small remains of carrion beneath the snow (Mullen and Pitelka 1972), access may become difficult when snow is too deep (Willebrand et al. 2017).
We did not observe any notable influence of small rodent abundance on red fox use of gut piles in the autumn. Our results suggest that red foxes utilize this resource equally between years, regardless of small rodent density. Gut piles are a pulsed but predictable resource, both in terms of abundance and distribution, compared to other carrion resources (Wikenros et al. 2013; Gomo et al. 2017). This may lead to behavioral adaptations (Tsukada 1997), where red foxes, independent of alternative prey abundance, actively search for gut piles when the moose hunting season begins.
An important limitation in our study design is that it is not possible to identify individual foxes on the photographs. For this reason, it is challenging to determine whether spatiotemporal variation in bait visitation is due to changes in abundance (NRH) or in the frequency of visits by individual foxes (FRH). Bait visitation is a product of both numerical and functional responses, and we cannot quantify their relative influence in each habitat separately. However, based on our predictions, we can address differences in the importance of the two responses by comparing spatiotemporal patterns in the two contrasting habitats.
In forests, our results give little support for numerical response, but support for functional responses, since scavenging at the bait increased in areas and periods with a low accessibility of main prey, i.e., during periods of low small rodent abundance. No signs of numerical responses at baits in forest may imply more stable and saturated red fox populations within this habitat. In alpine tundra habitats, fox visitation rates on baits were on average twice as high as in forest habitats, indicating that carrion in alpine tundra habitats may be an attractive, limited, and crucial resource independent of rodent phase, in contrast to the more productive forest areas. Habitats with highly fluctuating prey abundance are unlikely to support permanent high densities of red foxes, but abundance may increase markedly following peaks in prey abundance due to increased reproduction (Englund 1980). Space use of red foxes appears to be highly flexible, and adults as well as young frequently shift their home ranges or conduct exploratory trips (Walton et al. 2017). After rodent peak years, we observed a marked increase in activity on baits in alpine tundra, which could also imply an increase in density, either through higher reproduction or influx of foxes from neighboring forest areas attracted to vacant territories with high prey availability.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The codes used in R during the current study are available from the corresponding author on reasonable request.
References
Andreassen HP, Johnsen K, Joncour B, Neby M, Odden M (2020) Seasonality shapes the amplitude of vole population dynamics rather than generalist predators. Oikos 129(1):117–123
Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Grothendieck G, Green P, Bolker MB (2015) Package ‘lme4’. Convergence 12(1):2
Cagnacci F, Lovari S, Meriggi A (2003) Carrion dependence and food habits of the red fox in an Alpine area. Ital J Zool 70(1):31–38
Carricondo-Sanchez D, Samelius G, Odden M, Willebrand T (2016) Spatial and temporal variation in the distribution and abundance of red foxes in the tundra and taiga of northern Sweden. Eur J Wildl Res 62(2):211–218
Ehrich D, Schmidt NM, Gauthier G, Alisauskas R, Angerbjörn A, Clark K, Ecke F, Eide NE, Framstad E, Frandsen J (2020) Documenting lemming population change in the Arctic: can we detect trends? Ambio 49(3):786–800
Elmhagen B, Kindberg J, Hellstrom P, Angerbjorn A (2015) A boreal invasion in response to climate change? Range shifts and community effects in the borderland between forest and tundra. Ambio 44:39–50
Elmhagen B, Berteaux D, Burgess RM, Ehrich D, Gallant D, Henttonen H, Ims RA, Killengreen ST, Niemimaa J, Noren K, Ollila T, Rodnikova A, Sokolov AA, Sokolova NA, Stickney AA and Angerbjorn A (2017) Homage to Hersteinsson and Macdonald: climate warming and resource subsidies cause red fox range expansion and Arctic fox decline. Polar Res 36(sup1):3
Englund J (1970) Some aspects of reproduction and mortality rates in Swedish foxes (Vulpes vulpes), 1961-63 and 1966-69. Viltrevy 8:1–82
Englund J (1980) Population dynamics of the red fox (Vulpes vulpes L., 1758) in Sweden. The Red Fox, Springer: 107-121
Fletcher K, Aebischer NJ, Baines D, Foster R, Hoodless AN (2010) Changes in breeding success and abundance of ground-nesting moorland birds in relation to the experimental deployment of legal predator control. J Appl Ecol 47(2):263–272
Gallant D, Lecomte N, Berteaux D (2020) Disentangling the relative influences of global drivers of change in biodiversity: a study of the twentieth-century red fox expansion into the Canadian Arctic. J Anim Ecol 89(2):565–576
Gilg O, Kovacs KM, Aars J, Fort J, Gauthier G, Grémillet D, Ims RA, Meltofte H, Moreau J, Post E (2012) Climate change and the ecology and evolution of Arctic vertebrates. Ann N Y Acad Sci 1249(1):166–190
Gomo G, Mattisson J, Hagen BR, Moa PF, Willebrand T (2017) Scavenging on a pulsed resource: quality matters for corvids but density for mammals. BMC Ecol 17(1):22
Hagen BR (2014) Estimating ungulate carrion biomass and possible ecological effects on red fox (Vulpes vulpes) in central Norway. M.Sc., Hedmark University College
Hamel S, Killengreen ST, Henden JA, Eide NE, Rød-Eriksen L, Ims RA, Yoccoz NG (2013) Towards good practice guidance in using camera-traps in ecology: influence of sampling design on validity of ecological inferences. Methods Ecol Evol 4(2):105–113
Henden JA, Ims RA, Yoccoz NG (2009) Nonstationary spatio-temporal small rodent dynamics: evidence from long-term Norwegian fox bounty data. J Anim Ecol 78(3):636–645
Henden J-A, Stien A, Bårdsen B-J, Yoccoz NG, Ims RA, Hayward M (2014) Community-wide mesocarnivore response to partial ungulate migration. J Appl Ecol 51(6):1525–1533
Ims RA, Fuglei E (2005) Trophic interaction cycles in tundra ecosystems and the impact of climate change. Bioscience 55(4):311–322
Jahren T, Storaas T, Hagen B-R, Willebrand T, Fossland Moa P (2016) Declining reproductive output in capercaillie and black grouse – 16 countries and 80 years. Anim Biol 66(3-4):363–400
Jahren T, Odden M, Linnell JD, Panzacchi M (2020) The impact of human land use and landscape productivity on population dynamics of red fox in southeastern Norway. Mamm Res 65(3):503–516
Jędrzejewski W, Jędrzejewska B (1992) Foraging and diet of the red fox Vulpes vulpes in relation to variable food resources in Biatowieza National Park, Poland. Ecography 15(2):212–220
Kausrud KL, Mysterud A, Steen H, Vik JO, Østbye E, Cazelles B, Framstad E, Eikeset AM, Mysterud I, Solhøy T (2008) Linking climate change to lemming cycles. Nature 456(7218):93–97
Killengreen ST, Lecomte N, Ehrich D, Schott T, Yoccoz NG, Ims RA (2011) The importance of marine vs. human-induced subsidies in the maintenance of an expanding mesocarnivore in the arctic tundra. J Anim Ecol 80(5):1049–1060
Leroux SJ, Loreau M (2008) Subsidy hypothesis and strength of trophic cascades across ecosystems. Ecol Lett 11(11):1147–1156
Lindström ER, Hörnfeldt B (1994) Vole cycles, snow depth and fox predation. Oikos 70(1):156–160
Lowe S, Browne M, Boudjelas S, De Poorter M (2000) 100 of the world’s worst invasive alien species: a selection from the global invasive species database, Invasive Species Specialist Group Auckland, New Zealand
Marolla F, Aarvak T, Øien IJ, Mellard JP, Henden JA, Hamel S, Stien A, Tveraa T, Yoccoz NG, Ims RA (2019) Assessing the effect of predator control on an endangered goose population subjected to predator-mediated food web dynamics. J Appl Ecol 56(5):1245–1255
Moen A (1998) Nasjonalatlas for Norge: Vegetasjon. Hønefoss, Statens kartverk
Mullen DA, Pitelka FA (1972) Efficiency of winter scavengers in the Arctic. Arctic, 225–231
Needham R, Odden M, Lundstadsveen SK, Wegge P (2014) Seasonal diets of red foxes in a boreal forest with a dense population of moose: the importance of winter scavenging. Acta Theriol 59(3):391–398
Newsome TM, Dellinger JA, Pavey CR, Ripple WJ, Shores CR, Wirsing AJ, Dickman CR (2015) The ecological effects of providing resource subsidies to predators. Glob Ecol Biogeogr 24(1):1–11
Norwegian Meteorological Institute (NMI) (2019) eKlima: Free access to weather and climate data from Norwegian Meteorological Institute from historical data to real time observations, Norwegian Meteorological Institute.
Oro D, Genovart M, Tavecchia G, Fowler MS, Martínez-Abraín A (2013) Ecological and evolutionary implications of food subsidies from humans. Ecol Lett 16(12):1501–1514
Pasanen-Mortensen M, Pyykonen M, Elmhagen B (2013) Where lynx prevail, foxes will fail - limitation of a mesopredator in Eurasia. Glob Ecol Biogeogr 22(7):868–877
Pasanen-Mortensen M, Elmhagen B, Lindén H, Bergström R, Wallgren M, van der Velde Y, Cousins SA (2017) The changing contribution of top-down and bottom-up limitation of mesopredators during 220 years of land use and climate change. J Anim Ecol 86(3):566–576
Pereira LM, Owen-Smith N, Moleón M (2014) Facultative predation and scavenging by mammalian carnivores: seasonal, regional and intra-guild comparisons. Mammal Rev 44(1):44–55
Polis GA, Anderson WB, Holt RD (1997) Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu Rev Ecol Syst 28(1):289–316
Pozzanghera C, Sivy K, Lindberg M, Prugh L (2016) Variable effects of snow conditions across boreal mesocarnivore species. Can J Zool 94(10):697–705
Rød-Eriksen L, Skrutvold J, Herfindal I, Jensen H, Eide NE (2020) Highways associated with expansion of boreal scavengers into the alpine tundra of Fennoscandia. J Appl Ecol 57(9):1861–1870
Schipper J, Chanson JS, Chiozza F, Cox NA, Hoffmann M, Katariya V, Lamoreux J, Rodrigues AS, Stuart SN, Temple HJ (2008) The status of the world’s land and marine mammals: diversity, threat, and knowledge. Science 322(5899):225–230
Schmidt NM, Ims RA, Høye TT, Gilg O, Hansen LH, Hansen J, Lund M, Fuglei E, Forchhammer MC, Sittler B (2012) Response of an arctic predator guild to collapsing lemming cycles. Proc R Soc B Biol Sci 279(1746):4417–4422
Selås V, Vik JO (2006) Possible impact of snow depth and ungulate carcasses on red fox (Vulpes vulpes) populations in Norway, 1897-1976. J Zool 269(3):299–308
Sokolov AA, Sokolova NA, Ims RA, Brucker L, Ehrich D (2016) Emergent rainy winter warm spells may promote boreal predator expansion into the Arctic. Arctic 69(2):121–129
Sørensen OJ (2019) Population research on small rodents (Rodentia) and screws (Soridae) in Lierne Municipaility – Norway; 1988 – 2017 based on systematic catches with ordinary mouse traps in traplines. Version 1.12. N. University. Sampling event dataset doi:10.5281/zenodo.1295160 accessed via GBIF.org on 2020-08-05.
Sørensen OJ (2020) Population research on small rodents (Rodentia) and screws (Soridae) in Steinkjer Municipality – Norway; 1996-2017. N. University. GBIF.org (25 February 2020) GBIF Occurrence Download https://doi.org/10.15468/dl.cvih2e
Stoessel M, Elmhagen B, Vinka M, Hellström P, Angerbjörn A (2019) The fluctuating world of a tundra predator guild: bottom-up constraints overrule top-down species interactions in winter. Ecography 42(3):488–499
Tsukada H (1997) A division between foraging range and territory related to food distribution in the red fox. J Ethol 15(1):27–37
Tveraa T, Stien A, Bårdsen B-J, Fauchald P (2013) Population densities, vegetation green-up, and plant productivity: impacts on reproductive success and juvenile body mass in reindeer. PLoS One 8(2):e56450
Tylianakis JM, Didham RK, Bascompte J, Wardle DA (2008) Global change and species interactions in terrestrial ecosystems. Ecol Lett 11(12):1351–1363
Walton Z, Samelius G, Odden M, Willebrand T (2017) Variation in home range size of red foxes Vulpes vulpes along a gradient of productivity and human landscape alteration. PLoS One 12(4):e0175291
Wikenros C, Sand H, Ahlqvist P, Liberg O (2013) Biomass flow and scavengers use of carcasses after re-colonization of an apex predator. PLoS One 8(10):e77373
Willebrand T, Willebrand S, Jahren T, Marcström V (2017) Snow tracking reveals different foraging patterns of red foxes and pine martens. Mamm Res 62(4):331–340
Acknowledgements
We thank all students, local hunters, and rangers for their help in data collection. A special thanks to Pål Fossland Moa and Bjørn Roar Hagen at Nord University for their contributions with data collection in the forest habitat. Also, a special thanks to Ole Jakob Sørensen at Nord University for the use of small rodent data.
Funding
Open access funding provided by Inland Norway University Of Applied Sciences. The field work in the alpine areas was funded EU-LIFE through SEFALO+, EU/Interreg Sweden-Norway through Felles Fjellrev I and II (no. 20200939), and the Norwegian Environmental Agency. This study was part of the collaborative project ECOFUNC, funded by the Norwegian Research Council (NRC), grant no. 244557. The field work in forest areas was funded by Nord University.
Author information
Authors and Affiliations
Contributions
GG, JM, and MO conceived the idea of this study. GG, NEE, and LRE collected and compiled the data. GG, JM, and LRE performed statistical analyses. GG, JM, LRE, NEE, and MO all contributed in the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by: Dries Kuijper
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 13 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gomo, G., Mattisson, J., Rød-Eriksen, L. et al. Spatiotemporal patterns of red fox scavenging in forest and tundra: the influence of prey fluctuations and winter conditions. Mamm Res 66, 257–265 (2021). https://doi.org/10.1007/s13364-021-00566-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-021-00566-7