Abstract
Sexual dimorphism in anatomical traits has been widely studied in animals. Although pelvis dimorphism was mostly studied in humans, it occurs also in many other mammalian species. Here, we investigated sexual dimorphism in the pelvis of the bank vole Myodes glareolus using individuals with known sex and reproductive status of females (parous vs nulliparous). The analyses revealed that the size and shape of pelvis differed significantly between sexes, as well as between nulliparous and parous females. In comparison with males, females had a significantly longer pelvis and pubis bones and a longer obturator foramen length, but a smaller pubis width. Interestingly, the difference between parous and nulliparous females resembles that between females and males: parous females had bigger pelvis, which probably resulted from changes during pregnancy and after birth. Left bones were on average larger than right ones, but the magnitude of directional asymmetry was not different between sex and reproduction group. Moreover, we noticed that fluctuating asymmetry of pelvis and pubis length was higher in females than in males and higher in parous than in nulliparous females, what is presumably associated with locomotor performance. A discriminant function analysis performed for the four bone size traits showed that the traits can be effectively used for a nearly perfect recognition of sexes and also a quite reliable recognition of the reproductive status of females.
Similar content being viewed by others
Introduction
Sexual dimorphism in anatomical traits has been widely studied in animals. Among studies on osteological traits, sexual dimorphism of the pelvis (os coxa) has attracted the attention of several authors studying various mammalian species (Iguchi et al. 1995; Schutz et al. 2009a), including rodents (Trejo and Guthmann 2003; Berdnikovs et al. 2007; Balčiauskienė and Balčiauskas 2009a). Some of those studies have focused on pelvic development (Polaćek and Novotny 1969; Uesugi et al. 1992) and others on morphology (St. Clair 2007; Schutz et al. 2009a). Mammalian pelvis is a compound structure consisting of the two pubis bones (os pubis), two ilium bones (os ilium), and two ischium bones (os ischii). In most mammalian species, it varies across the sexes (Berdnikovs et al. 2007) and, generally, females have relatively larger pelvises than males (Tague 2005). Sexual dimorphism, with regard to size and shape of the pelvis, exists even where there is a small difference in size between males and females (Schutz et al. 2009a).
Pelvic dimorphism is primarily genetically determined and grows in response to sex hormones during puberty (Schutz et al. 2009b). It is thought that pelvic dimorphism may emerge also from functional pressures on the pelvis produced by weight bearing, locomotion, and parturition (Schutz et al. 2009b). Weight bearing affects the shape of the pelvis due to the pressures of weight to the rear limb. Animals with strong, thick legs use to have small pelvises, while those with thin limbs have larger pelvis (Carrier et al. 2005). Locomotion affects pelvic form through demand for limb orientation and muscle attachment (Carrier et al. 2005). Finally, anatomical changes are associated with pregnancy and parturition, resorption and remodelling edge of the pubic bone (Putschar 1976), losses or thickening of pubic bone (Ubelaker and De La Paz 2012), the extension of the pubic tubercle (Cox and Scott 1992), and changes in the pubic symphysis area (Angel 1969).
Pregnancy and parturition are one of the most important factors that influence the form of female pelvis (Johnson et al. 1988; Tague 1990; Cox and Scott 1992). The main function of female pelvis shape is to enable the passage of young through the aperture. Changes in the shape of the os coxae appear as a result of the first parturition event, and these transformations correlated with the relative size of individual offspring (Schutz et al. 2009a). Consequently, both maternal and neonatal sizes seemed to be very significant factor responsible for alterations to the female pelvis throughout parturition (Leutenegger 1974; Berdnikovs 2005; Tague 2005).
Paired quantitative traits can reveal asymmetry, especially directional and fluctuating (Leamy 1984). Directional asymmetry (DA) is the consistent difference between pairs of structures (the mean of one side is significantly larger than the mean of the other side). It has been widely studied throughout the animal kingdom, also in case of skeletal structures: e.g. of the stickleback fish Gasterosteus aculeatus (Bell et al. 2007), the pelvis of the cane toad Bufo marinus (Robins and Rogers 2002), the hind limbs of Agamidae lizards (Seligmann 1998), the limb bones of the harbour porpoise Phocoena phocoena (Galatius 2005), the mandible of the mouse Mus musculus (Leamy et al. 2000), and the skull and pelvis of the silver fox Vulpes vulpes (Kharlamova et al. 2010), macaque Macaca mulatta (Falk et al. 1988), and human Homo sapiens (Auerbach and Ruff 2006). On the other hand, fluctuating asymmetry (FA) reflects small random errors that occur during the development of a trait (Graham et al. 2010) and is often used as a measure of developmental instability. Thus, an increased FA may reflect a decreased heterozygosity and signal a compromised health and fitness (Dongen et al. 1999). On the other hand, a decreased asymmetry, both directional and fluctuating, may indicate a selection acting on locomotor performance traits (Garland and Freeman 2005).
Moreover, osteological measurements are also useful for the understanding of the ecology of a population of a particular species and, particularly, for assessing susceptibility to predation (Zalewski 1996). In many predatory birds and mammals, some bones of their prey resist digestion and are regurgitated as pellets and/or scats. These bones can be used, among other things, to estimate prey biomass (Raczyński and Ruprecht 1974; Borowski et al. 2008). Several studies have focused on the prediction of body weight based on measurements of the skull in the bank vole (e.g. Balčiauskienė and Balčiauskas 2009b), but not such estimations exist for pelvis bones. However, using pelvis measurements is a potentially very useful method to analyse the population structure of prey, because pelvis bones typically persist well in predators’ digestive tracks and, therefore, can be used later to take detailed measurements (Raczyński and Ruprecht 1974).
The main aim of this study was to investigate sexual dimorphism and symmetry of the pelvic bone and to check whether the structure of the pelvic bone differs between parous and nulliparous females of the bank vole. In addition, we will check whether the measurements of the pelvic bones can be practically applied to identify the sex and reproductive category of females.
Material and methods
The study was performed on the bank vole Myodes (Clethrionomys) glareolus, a common European small rodent which has been one of the most intensively studied species of wild rodents (e.g. Bujalska and Hansson 2000). The animals were captured in the Niepołomice Forest near Kraków (Southern Poland) in Aug.–Sept. 2000 and Sept.–Nov. 2001 and were used as a founding group for a permanent colony established in the Institute of Environmental Studies of the Jagiellonian University, as a basis for further research on the quantitative genetics of physiological parameters and a selection experiment (e.g. Sadowska et al. 2005, 2008, 2009). The animals were maintained in standard plastic mouse cages with sawdust bedding, at constant temperature (20 ± 2 °C) and photoperiod (16:8 h L/D). Water and food (a standard rodent chow of 24% protein, 3% fat, and 4% fibre) were provided ad libitum (Sadowska et al. 2005). Individuals used for the osteological morphometric analyses (N = 187, including 106 females and 81 males) were killed by cervical dislocation between February and May of the next year after the trapping or died within that period, so at the time of death, they were at least 6–10 months old. Some of the animals reproduced in the laboratory before they were killed.
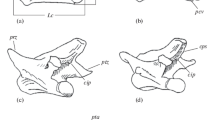
The corpses of the voles were stored at about −20 °C until 2012, but within that period, they got partly defrosted two times because of the power failure. The frozen corpses were put into the cotton net and subjected to the biological maceration in the laboratory incubator at 45 °C (Simonsen et al. 2011). Isolated and completely cleaned skeletons were dried and preserved in the plastic cans. All specimens (106 females and 81 males) were measured by one person (A.M.) using a digital calliper with accuracy of 0.01 mm. The following measurements were taken (Fig. 1):
-
LPEL, LPER—total length of the pelvis [indexes: L (left) and R (right)]
-
LOFL, LOFR—length of the obturator foramen (foramen obturatum)
-
LPUL, LPUR—the greatest length of the pubis
-
WPUL, WPUR—width of the pubis measured at the thinnest point of the ramus cranialis ossis pubis.
The measurements were performed twice (in about 6-week intervals) for each individual, in an order randomized with respect to year of origin, sex, and reproductive status. Based on whether a female reproduced in laboratory, it was classified operationally as “nulliparous” (has not given birth: N = 54) or “parous” (has given birth: N = 52). The parous females gave one or two litters (thus, primiparous and multiparous females were pooled in one group). Some of the females, those classified as both nulliparous and parous, could have also reproduced in the field before they were trapped (unfortunately, reproductive status of individuals was not recorded at the moment of trapping). However, because the trappings were performed in the autumn, most of the individuals were captured as subadults, whereas adult, reproductively active females were typically pregnant and gave birth in the laboratory after the trapping. Thus, the proportion of females misclassified as nulliparous was probably low. We will consider consequences of the possible misclassification in the “Discussion”. Statistical analyses were performed with SAS 9.3 for Windows (SAS Institute). In the first part of the analyses, we applied univariate linear mixed models (MIXED procedure with residual maximum likelihood estimation). The dependent variables were four morphometric parameters (LPE, LPO, LPU, and WPU), each analysed separately. Each individual was represented by four observations: left and right sides, two replicates of each. We first estimated simple models, which included only the fixed effect of “side” (left or right) and random effects of “individual” and side × individual interaction. Results of the analyses provided information about directional and fluctuating asymmetry, and repeatability of the trait measurements (Graham et al. 2010). However, we used the results only to assess the repeatability, quantified as the coefficient of intraclass correlation (r i), i.e. the ratio of individual variance to the sum of all variance components, including residual variance (e.g. Graham et al. 2010; Nakagawa and Schielzeth 2010).
Next, the same analytical approach was extended to address in one model the questions about the (a) effect of sex-and-reproductive status, (b) consistency of the traits across 2 years, (c) DA, (d) FA, and (e) repeatability of the bone size measurements (now considering that the animals do not represent one random sample). The independent fixed factors were “sex-and-reproductive status” (SRS, with three groups: nulliparous females, parous females, and males), “year” (2000 or 2001), side (left or right), and interactions between the three factors. The random effects were individual (nested in SRS and Year) and side × individual interaction. In this model, the fixed effect of side describes the directional asymmetry, the random side × individual interaction quantifies the fluctuating asymmetry, and the residual variance represents the measurement error (Graham et al. 2010). In addition, SRS × side and year × side interactions inform whether the directional asymmetry differed between the year or SRS groups.
To identify a model with the appropriate random effects structure (Littell et al. 2006), in preliminary analyses, we tested 16 alternative models: both the individual and side × individual random effects were modelled with either common variances for the entire dataset or separate variances for SRS groups or separate for year groups or separate for SRS × year subgroups. Thus, there were four variants for each of the two random effects, i.e. a total of 16 combinations. The preliminary analyses allowed to find the most relevant variance structure for the final analyses based on the Akaike Information Criterion (AIC) but enabled also performing a likelihood ratio (LR) test to check formally whether the individual variation or fluctuating asymmetry differed between year or SRS groups (Littell et al. 2006). The LRs were computed from the difference between log-likelihoods of models with a more complex and a simpler variance structure and were compared with chi-square distribution with degrees of freedom equal to the difference in the number of estimated variance components.
Fixed effects in the final models were tested by F tests, and Satterthwaite approximation for non-orthogonal models was applied to obtain the denominator degrees of freedom (Satterthwaite option in the Model command of SAS MIXED procedure). The analyses were followed by a set of two planned orthogonal contrasts for the SRS factor: (1) combined females vs males and (2) nulliparous vs parous females.
The above analytical approach, although recommended as the most appropriate tool for analysing asymmetry (Graham et al. 2010), could lead to dubious conclusions concerning differences in the asymmetry between sexes and other groups, if the groups differ also in the mean value of the bone size. For example, if a bone is, on average, larger in males than in females, it is reasonable to suspect that both the mean difference between the right and left sides (DA) and individual differences between the sides (FA) could be also larger in males, because the magnitude of the differences is likely to be proportional to the mean of the trait. To address such a problem, some researchers advise to perform analyses on the differences between the right and left sides expressed as a percentage of the mean value (e.g. Dongen 2006). In the second part of analyses, we applied a similar approach to adjust the magnitude of DA and FA for differences in mean values, but within the framework of the mixed model analyses recommended by Graham et al. (2010), such as described above. Thus, the analysis was performed for individual bone measurements (not on a difference between the right and left bones), and therefore, each individual was represented by four values (right and left bones, each measured twice), but the values were deviations of the particular bone measurement from the mean of a given individual, expressed as the percentage of the mean. The model included fixed effects of side, side × SRS and side × year, and random side × individual interaction. However, because the mean values of the deviations are (by definition) zero, the model did not include the random effect of individual (this variance must be zero). As the means for each individual are zero, also all the mean groups of individuals must be equal zero, and consequently, the fixed effects of SRS, year, and SRS × year interactions must be zero, too. However, the terms were retained in the model for technical reason (MIXED procedure allows estimating separate variance components only according to categorical variables present in the model). As in the models for absolute measurements, in preliminary analyses, we tested models with four alternative variance structures of the side × individual random effect (common and grouped by SRS, year, or both SRS and year). Model selection and tests of hypotheses were performed as described above.
It could be argued that in all the above analyses the “replicate number” (the first vs the second measurement) should be included as another fixed factor in the mixed model (i.e. that a repeated measures model should be implemented). Including such a factor would be certainly justified if the replicated measurements were performed on alive animals and the trait of interest could indeed change with time. However, in the case of our data, the difference between replicates results solely from the measurement error. Therefore, including the replicate number in the model would effectively remove a part of the measurement error that changes systematically with time (i.e. “drift”) and would effectively lead to underestimation of the total contribution of measurement error (represented by residual term in the mixed models). Consequently, both the repeatability and relative contribution of fluctuating asymmetry to the total variance would be overestimated. However, additional models with the replication number included as another effect showed that the difference between the two replications explains only a negligible percentage of total variance (LPE 0.09%, LOF 0.00%, LPU 0.13%, WPU 0.15%), so the decision of including or not the additional factor had no practical consequences. Note also that, because within the framework of the implemented models the effects of fixed factors are estimated for values “internally” averaged across the measurements replicated in a given individual, including or not including the fixed effect of replicate has no influence on either the estimation of the effect size or inferences concerning significance of the effects.
Finally, to check whether the four bone size parameters can be practically used to determine the sex-and-reproductive status of bank voles, we applied a discriminant function analysis. For this analysis, we used the SAS DISRCIM procedure with parametric discriminant function and pooled covariance structure (a model with separate variances for groups gave the same predictions, but its presentation is less transparent). To simulate more realistic conditions, such as encountered in an attempt of using bones recovered from pellets regurgitated by raptors, in this analyses for each individual vole, we randomly chosen the size of either left or right bone (the mean from two replicated measurements). The effectiveness of the discriminant function was assessed as the proportion of individuals correctly assigned to their proper sex-and-reproductive status categories in a cross-validation procedure implemented in the DISCRIM procedure: for each individual, a separate discriminant function is computed based on all other individuals, and the function is used to predict the category of the particular individual (not used for constricting the discriminant function). Canonical discriminant function scores were also calculated to illustrate the distribution of the data points described by the four morphometric parameters is a space reduced to two dimensions that allowed the best separation of the four SRS groups.
Results
The tables with complete descriptive statistics of the left and the right sizes of the bones are presented in the electronic supplementary material (Table S1). In the main text, we present in detail only the results from mixed models and focus on the three parameters of interest (mean bone size, directional asymmetry, and fluctuating asymmetry).
Simple mixed models (not including the effects of SRS or year; Supporting Information Appendix S2) showed a very high repeatability of the measurements of the pelvis length (LPE, r i = 0.99), obturator foramen length (LOF, r i = 0.98), pubis length (LPU, r i = 0.98), and pubis width (WPU, r i = 0.97) (Fig. 2). The small differences in repeatability result primarily from differences in mean values of the traits (Fig. 3), which inevitably results in differences in the relative magnitude of the measurement error. Thus, the contribution of measurement error, quantified as the proportion of residual variance to the total variance, was lowest for LPE (0.2%), intermediate for LOF (1.0%) and LPU (0.8%), and highest for WPU (1.9%). The contribution of FA, quantified as the proportion of side × individual variance in total variance, was slightly higher for LOF (1.1%) and WPU (0.9%) than for the pelvis or pubis length (LPE 0.7%, LPU 0.8%). The simple models indicated also a small but significant directional asymmetry (the left size values were higher than the right ones; Fig. 3), but we will discuss the issue in the context of more complex models, which take into account also the differences between sex-and-reproductive status groups (SRS) and two sampling years.
Graphical presentation of the repeatability of the four bone measurements (results of measurement 2 plotted against results of measurement 1, performed about 6 weeks earlier): length of the pelvis (LPE), length of the obturator foramen (LOF), length of the pubis (LPU), and width of the pubis (WPU). Symbols distinguished: circles nulliparous females, squares parous females, triangles males; open symbols left side, closed symbols right side. Diagonal line is the identity line
Comparison of the mean values of the right and left bone sizes in the three “sex-and-reproductive status” (SRS) groups: nulliparous (N = 54) and parous (N = 52) females and males (N = 81). LPE length of the pelvis, LOF length of the obturator foramen, LPU length of the pubis, WPU × 10 width of the pubis × 10 (multiplied for the purpose of the graph presentation). The results are presented as the adjusted least square means ± SE for SRS × side subgroups, obtained from the mixed ANOVA model (see “Material and methods”)
Results of analyses of the complex mixed models with alternative variance structure, followed by LR tests, showed that both individual variance and FA (represented by side × individual random interaction) differed between SRS groups in each of the traits (Supporting Information Appendix S3). The individual variance of LPE, LOF, and LPU was lowest in males, intermediate in parous females, and highest in nulliparous females, whereas the pattern was reversed for WPU (Supporting Information Appendix S3). In the case of LOF and LPU, separating variances for 2 years also resulted in model improvement, and the best models had six separate estimates of variance components, for the six combinations of SRS × year subgroups. In both traits, the individual variance in females was higher in 2001 than in 2000, whereas in males, the trend was reversed (but differences between the years were smaller). FA variance of LPE, LOF, and LPU was also lowest in males, but it was highest in parous females and intermediate in nulliparous females. Again, the pattern was reversed for WPU, for which, the FA variance was lowest in females (actually, the estimate was negative and, therefore, fixed to zero; Supporting Information Appendix S3).
The comparisons of alternative models lead to conclusion that in final analyses, we should use models with individual and side × individual variances separate for SRS groups, and in the case of LOF and LPU, the individual variance should be also separated for year groups (but results of all the alternative models lead to the same conclusions concerning fixed effects). Each of the four traits differed highly significantly between the SRS groups (p < 0.0001, Fig. 3, Supporting Information Appendix S3). The mean values of LPE, LOF, and LPU were lowest in males, intermediate in nulliparous females, and highest in parous females, whereas the pattern was reversed for WPU (Fig. 3). Orthogonal contrasts showed that in all the four traits, both the differences between males and combined females, and between parous and nulliparous females, were highly significant (p < 0.0001 for all comparisons). Interestingly, for LOF and LPU, the difference between nulliparous and parous females was as large as that between nulliparous females and males, and for LPE, the difference between the two female groups (1.3 mm) was even markedly larger than that between nulliparous females and males (0.6 mm; Fig. 3).
The analysis showed also a significantly directional asymmetry. All the traits tended to be larger on the left than on the right side, although the mean difference was only about 0.02 mm for the bone lengths (LPE: p = 0.08, LOF: p = 0.002, LPU: p = 0.005) and about 0.01 mm for WPU (p = 0.025, Fig. 3, Table S1). For WPU, the results were complicated by highly significant effects of year (LSM ± SE in 2000: 0.42 ± 0.008 mm, in 2001: 0.46 ± 0.008; p = 0.002) and side × year interaction (p = 0.001: the difference between the left and right sides was present only in 2001). The effects of year or interaction terms were not significant in three bone lengths. Importantly, the SRS × side interaction was not present, which shows that the tendency of directional asymmetry did not differ between the sex-and-reproductive status groups.
In the analyses reported above, we reported that FA variance differed between SRS groups, but, as we explained in the “Material and methods”, the result could be an artefact resulting from differences between SRS groups in the mean values of the traits. Therefore, we analysed also similar models in which each measurement was expressed as a proportional (%) deviation from the mean of four measurements of a given individual (Supporting Information Appendix S4). The analyses confirmed that FA variance of LPE and LPU was lowest in males, intermediate in nulliparous females, and highest in parous females (LPE: χ 2 = 22.7, df = 2, p < 0.0001; LPU: χ 2 = 28.5, df = 2, p < 0.0001; Supporting Information Appendix S4). For LOF, the differences were not significant (χ 2 = 3.2, df = 2, p = 0.20), whereas in the case of WPU, the FA variance component was so small that the estimate was actually negative, and fitting separate variances for SRS groups did not improve the model (χ 2 = 2.7, df = 2, p = 0.26). However, for both LOF and WPU, the results showed a tendency for higher FA in females than in males. The results confirmed also the presence of a small but statistically significant directional asymmetry in LPE (p = 0.009), LOF (p < 0.0001), and LPU (p < 0.0001): all the trait values were larger on the left side (Supporting Information Appendix S4). In WPU, there was also such a trend (p = 0.006), but the pattern was complicated by highly significant interactions (SRS × side: p = 0.003; year × side: p < 0.0001; SRS × year × side: p = 0.006), which practically preclude any general conclusions.
The discriminant function analysis showed that the four bone size parameters allowed a nearly perfect assignment of individuals as females and males: all females were properly recognized as females, and only three males (3.7%) were misrecognized as females (Table 1, Fig. 4). Moreover, 80% of females were properly assigned to parous vs nulliparous groups. Canonical analysis (Fig. 4) showed that males were discriminated clearly from females by the first canonical variable, which had high positive loadings for the three length parameters (LPE 0.67, LOF 0.80, and LPU 0.85) and a highly negative for pubis width (WPU −0.98). The difference between nulliparous and parous females was underlined by both the first and the second canonical variables, which was determined nearly entirely by the three length parameters (loadings for LPE 0.72, LOF 0.50, and LPU 0.47), with no remarkable contribution of pubis width (WPU 0.13). The first canonical discriminant variable accounted for 96.5% of variance in the four bone parameters that distinguish the three groups, and the second variable only 3.5%, but both are highly significant predictors of group membership (canonical correlation ± SE: first = 0.92 ± 0.01, F 8,362 = 86.2, p < 0.0001; second = 0.41 ± 0.06, F 3,182 = 13.2, p < 0.0001).
The plot of two canonical discriminant variables scores for the three “sex-and-reproductive status” (SRS) groups of bank voles (see the “Results” section for information on original traits’ contribution to the canonical variables)
Discussion
Our analyses revealed significant differences in the size of pelvis bones between the sexes. Overall, we found that females of bank vole have larger pelvis than males. Females tend to have longer and thinner pubis bone than males, which may result from hormone effects during puberty (Gardner 1936; Uesugi et al. 1992; Iguchi et al. 1995). Hormones, especially estrogens, affect changes in the structure and shape of the pelvis (Berdnikovs et al. 2007). Differences in the length of the pubis may be related to the reproductive status and size of mother and her offspring (Balčiauskienė and Balčiauskas 2009a). Larger offspring size requires the extension of the birth canal which leads to the lengthening of pubic bone (Schultz 1949; Leutenegger 1974; Mobb and Wood 1977; Ridley 1995; Berdnikovs 2005). Similarly as in the bank voles, in the house mouse, pubic bone is more elongated, more slender, and thinner in females than in males (Gardner 1936).
The multiparous females may obtain extreme remodelling of the pubic bone, including elongation and resorption of the pubic symphysis (Brown and Twigg 1969). The mineral cost of the mother during pregnancy and lactation increase as the foetus draws from maternal stores due to the mineralization of the developing skeleton (Bowman and Miller 1999). Especially, if dietary sources are limited, greater amounts is provided from skeletal stores of mother, as was described in rats (Bowman and Miller 1999), cows (Johanson and Berger 2003), and humans (Specker and Binkley 2005). We also found that the differences in bone size of the pelvis occur not only between females and males but also between nulliparous and parous females. Parturition may affect the size of various skeletal elements (Bowman and Miller 1999, 2001; Johanson and Berger 2003; Specker and Binkley 2005), but relatively little is known about the effects of parturition event on the pelvis dimorphism in small mammals (Schutz et al. 2009b). Our study showed that parous females revealed more profound differences in pubic size from males than nulliparous females. The difference is presumably primarily due to birth (Schilling 2005) and energy requirement of reproducing females (Kaczmarski 1966; Sadowska et al. 2016).
Information on the size and shape of the pelvis in many animal species are of great importance in many fields of ecology (Trejo and Guthmann 2003), morphology (West 1990; Morris 2008), and palaeontology (Ratnikov 2001).
Differences in fluctuating asymmetry (FA) between the studied traits are very important to understand the movement processes of mammals because it is clear that more important are consequences in the asymmetry of bone size than in the obturator foramen (a hole inside the bone). Directional asymmetry (DA) and FA decrease locomotor abilities. FA is negatively associated with racing ability among individual thoroughbred horses (Manning and Ockenden 1994) and with sprint spend in femur lizards (Martin and Lopez 2001). Although we did not find any study on similar processes in rodents, they are well studied in humans (Al-Eisa et al. 2006). Our results showed that FA variance of LPE, LOF, and LPU was lowest in males, intermediate in nulliparous females, and highest in parous females. Lower FA in males than in females agrees with the hypothesis that, for males, operates stronger selection on locomotor performance, whereas enlarged FA in female after parturition, which may be the result of reproduction (females investing in birth), worsen their performance locomotor. Observing the locomotor performance, also of big importance is having DA (Garland and Freeman 2005).
Note that, as we have mentioned in the “Material and methods”, some of the females operationally treated in this analysis as nulliparous could, in fact, reproduce in the field before they were trapped. In fact, the plot of canonical discriminant scores shows a group of eight females from the nominally nulliparous group with the canonical scores very different from the rest of the nulliparous females (high values of both the first and the second canonical scores). Thus, these females could, in fact, have reproduced before they were captured. The results support the possibility of using osteological information in field studies with animals with no known age. The results of our study provide also an opportunity (however, not tested through the paper) to establish sexual reproductive status of the bank vole, captured by diurnal raptor and owls (Raczyński and Ruprecht 1974; Zalewski 1996). Advanced molecular techniques are currently being used to confirm species diagnostics and even the sex structure of consumed prey by raptors (Buś et al. 2013), and added classical osteological methods will enlarge information on the reproductive status of females.
References
Al-Eisa E, Egan D, Deluzio K, Wassersug R (2006) Effects of pelvic asymmetry and low back pain on trunk kinematics during sitting: a comparison with standing. Spine 31:135–143
Angel JL (1969) The bases of paleodemography. Am J Phys Anthropol 30:427–438
Auerbach BM, Ruff CB (2006) Limb bone bilateral asymmetry: variability and commonality among modern humans. J Hum Evol 50:203–218
Balčiauskienė L, Balčiauskas L (2009a) On pelvis morphometry of the root vole Microtus oeconomus. Vet Med Zoothec 46:3–9
Balčiauskienė L, Balčiauskas L (2009b) Prediction of the body mass of the bank vole Myodes glareolus from skull measurements. Est J Ecol 58:77–85
Bell MA, Khalef V, Travis MP (2007) Directional asymmetry of pelvic vestiges in threespine stickleback. J Exp Zool 308B:189–199
Berdnikovs S (2005) Evolution of sexual dimorphism in Mustelids. Doctoral thesis. Cincinnati, Ohio: University of Cincinnati
Berdnikovs S, Bernstein M, Metzler A, German RZ (2007) Pelvic growth: ontogeny of size and shape sexual dimorphism in rat pelvis. J Morphol 268:12–22
Borowski Z, Keller M, Włodarska A (2008) Applicability of cranial features for the calculation of vole body mass. Ann Zool Fenn 45:174–180
Bowman BM, Miller SC (1999) Skeletal mass, chemistry, and growth during and after multiple reproductive cycles in the rat. Bone 25:553–559
Bowman BM, Miller SC (2001) Skeletal adaptations during mammalian reproduction. J Musculoskelet Neuronal Interact 1:347–355
Brown JC, Twigg GI (1969) Studies on the pelvis in British Muridae and Cricetidae (Rodentia). J Zool (Lond) 158:81–132
Bujalska G, Hansson L (2000) Bank vole biology: recent advances in the population biology of a model species. Pol J Ecol 48:1–235
Buś MM, Żmihorski M, Romanowski J, Balciauskiene L, Cichocki J, Balciauskas L (2013) High efficiency protocol of DNA extraction from Micromys minutus mandibles from owl pellets: a tool for molecular research of cryptic mammal species. Acta Theriol 59:99–109
Carrier DR, Chase K, Lark KG (2005) Genetics of canid skeletal variation: size and shape of the pelvis. Genome Res 15:1825–1830
Cox M, Scott A (1992) Evaluation of the obstetric significance of some pelvic characters in an 18th century British sample of known parity status. Am J Phys Anthropol 89:431–440
Dongen SV (2006) Fluctuating asymmetry and developmental instability in evolutionary biology: past, present and future. J Evol Biol 19:1727–1743
Dongen SV, Sprengers E, Löfstedt C, Matthysen E (1999) Heritability of tibia fluctuating asymmetry and developmental instability in the winter moth (Operophtera brumata L.) (Lepidoptera, Geometridae). Heredity 82:535–542
Falk D, Pyne L, Helmkamp RC, Derousseau CJ (1988) Directional asymmetry in the forelimb of Macaca mulatta. Am J Phys Anthropol 77:1–6
Galatius A (2005) Bilateral directional asymmetry of the appendicular skeleton of the harbor porpoise (Phocoena phocoena). Mar Mamm Sci 21:401–410
Gardner WU (1936) Sexual dimorphism of the pelvis of the mouse, the effect of estrogenic hormones upon the pelvis and upon the development of scrotal hernias. Am J Anat 59:459–483
Garland T Jr, Freeman PW (2005) Selective breeding for high endurance running increases hindlimb symmetry. Evolution 59:1851–1854
Graham JH, Raz S, Hel-Or H, Nevo E (2010) Fluctuating asymmetry: methods, theory, and applications. Symmetry 2:466–540
Iguchi T, Fukazawa Y, Bern HA (1995) Effects of sex hormones on oncogene expression in the vagina and on development of sexual dimorphism of the pelvis and anococcygeus muscle in the mouse. Environ Health Perspect 103:79–82
Johanson JM, Berger PJ (2003) Birth weight as a predictor of calving ease and perinatal mortality in Holstein cattle. J Dairy Sci 86:3745–3755
Johnson SK, Deutscher GH, Parkhurst A (1988) Relationships of pelvis structure, body measurements, pelvic area and calving difficulty. J Anim Sci 66:1081–1088
Kaczmarski F (1966) Bioenergetics of pregnancy and lactation in the bank vole. Acta Theriol 19:409–417
Kharlamova AV, Trut LN, Chase K, Kukekova AV, Lark KG (2010) Directional asymmetry in the limbs, skull and pelvis of the silver fox (V. vulpes). J Morphol 271:1501–1508
Leamy L (1984) Morphometric studies in inbred and hybrid house mice. V. Directional and fluctuating asymmetry. Am Nat 123:579–593
Leamy LJ, Pomp D, Eisen EJ, Cheverud JM (2000) Quantitative trait loci for directional but not fluctuating asymmetry of mandible characters in mice. Genet Res 76:27–40
Leutenegger W (1974) Functional aspects of pelvic morphology in simian primates. J Hum Evol 3:207–222
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) In: 2nd edn (ed) SAS® for mixed models. SAS Institute Inc, Cary
Manning JT, Ockenden L (1994) Fluctuating asymmetry in racehorses. Nature 370:185–186
Martin J, Lopez P (2001) Hindlimb asymmetry reduces escape performance in the lizard Psammodromus aldirus. Physiol Biochem Zool 74:619–624
Mobb GE, Wood BA (1977) Allometry and sexual dimorphism in the primate innominate bone. Am J Anat 150:531–537
Morris P (2008) A review of mammalian age determination methods. Mammal Rev 2:69–104
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956
Polaćek P, Novotny VL (1969) Sex differences of the bony pelvis in growing macaques. Folia Morphol (Praha) 13:145–157
Putschar WGJ (1976) The structure of the human symphysis pubis with special consideration of parturition and its sequelae. Am J Phys Anthropol 45:589–594
Raczyński J, Ruprecht AL (1974) The effect of digestion on the osteological composition of owl pellets. Acta Ornithol 14:25–38
Ratnikov VY (2001) Osteology of Russian toads and frogs for paleontological researches. Acta Zool Cracov 44:1–23
Ridley M (1995) Pelvic sexual dimorphism and relative neonatal brain size really are related. Am J Phys Anthropol 97:197–200
Robins A, Rogers LJ (2002) Limb preference and skeletal asymmetry in the cane toad, Bufo marinus (Anura: Bufonidae). Laterality 7:261–275
Sadowska ET, Labocha MK, Baliga K, Stanisz A, Wróblewska AK, Jagusik W, Koteja P (2005) Genetic correlations between basal and maximum metabolic rates in a wild rodent: consequences for evolution of endothermy. Evolution 59:672–681
Sadowska ET, Baliga-Klimczyk K, Chrzascik KM, Koteja P (2008) Laboratory model of adaptive radiation: a selection experiment in the bank vole. Physiol Biochem Zool 81:627–640
Sadowska ET, Baliga-Klimczyk K, Labocha MK, Koteja P (2009) Genetic correlations in a wild rodent: grass-eaters and fast-growers evolve high basal metabolic rates. Evolution 63:1530–1539
Sadowska ET, Król E, Chrzascik KM, Rudolf AM, Speakman JR, Koteja P (2016) Limits to sustained energy intake. XXIII. Does heat dissipation capacity limit the energy budget of lactating bank voles? J Exp Biol 219:805–815
Schilling N (2005) Ontogenetic development of locomotion in small mammals—a kinematic study. J Exp Biol 208:4013–4034
Schultz AH (1949) Sex differences in the pelvis of primates. Am J Phys Anthropol 7:401–424
Schutz H, Polly PD, Krieger JD, Guralnick RP (2009a) Differential sexual dimorphism: size and shape in the cranium and pelvis of grey foxes (Urocyon). Biol J Linn Soc 96:339–353
Schutz H, Donovan ER, Hayes JP (2009b) Effects of parity on pelvic size and shape dimorphism in Mus. J Morphol 270:834–842
Seligmann H (1998) Evidence that minor directional asymmetry is functional in lizard hindlimbs. J Zool 245:205–208
Simonsen KP, Rasmussen AR, Mathisen P, Petersen H, Borup F (2011) A fast preparation of skeletal materials using enzyme maceration. J Forensic Sci 56:480–484
St. Clair EM (2007) Sexual dimorphism in the pelvis of Microcebus. Int J Primatol 28:1109–1122
Specker B, Binkley T (2005) High parity is associated with increased bone size and strength. Osteoporos Int 16:1969–1974
Tague RG (1990) Morphology of the pubis and preauricular area in relation to parity and age at death Macaca mulatta. Am J Phys Anthropol 82:517–525
Tague RG (2005) Big-bodied males help us recognize that females have big pelves. Am J Phys Anthropol 127:392–405
Trejo A, Guthmann N (2003) Owl selection on size and sex classes of rodents: activity and microhabitat use of prey. J Mammal 84:652–658
Ubelaker DH, De La Paz JS (2012) Skeletal indicators of pregnancy and parturition: a historical review. J Forensic Sci 57:866–872
Uesugi Y, Taguchi O, Noumura T, Iguchi T (1992) Effects of sex steroids on the development of sexual dimorphism in mouse innominate bone. Anat Rec 234:541–548
West B (1990) A tale of two innominates. Circa 6:107–114
Zalewski A (1996) Choice of age classes of bank voles Clethrionomys glareolus by pine marten Martes martes and tawny owl Strix aluco in Białowieża National Park. Acta Oecol 17:233–244
Acknowledgements
We thank several students and technicians, especially Marta Labocha, Aleksandra Wróblewska, and Katarzyna Baliga-Klimczyk for their help with the animal trapping and maintenance and Koralia and Zbigniew Ostrega for the English proofreading. This research was funded by a grant from the Polish Ministry of Science and Higher Education (no. NN 304375238) and internal university grant (no. DS/WBINOZ/INOS/757).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animal care procedures were approved by the State Ethical Committee for Experiments on Animals, Poland (DB/KKE/PL-111/2001).
Funding
This study was funded by a grant from the Polish Ministry of Science and Higher Education (no. NN 304375238, to P.T.) and grant (no. DS/WBINOZ/INOS/757, to P.K.) from Jagiellonian University.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by: Andrzej Zalewski
Electronic supplementary material
Table S1
(DOCX 17 kb)
Supporting Information Appendix S2
(XLSX 22 kb)
Supporting Information Appendix S3
(XLSX 42 kb)
Supporting Information Appendix S4
(XLSX 25 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Matysiak, A., Malecha, A.W., Jakubowski, H. et al. Sexual dimorphism, asymmetry, and the effect of reproduction on pelvis bone in the bank vole, Myodes glareolus . Mamm Res 62, 297–306 (2017). https://doi.org/10.1007/s13364-017-0317-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-017-0317-1