Abstract
The pursuit of high-throughput sample analysis from complex matrix demands development of multiple ionization techniques with complementary specialties. A versatile integrated ambient ionization source (iAmIS) platform is proposed in this work, based on the idea of integrating multiple functions, enhancing the efficiency of current ionization techniques, extending the applications, and decreasing the cost of the instrument. The design of the iAmIS platform combines flowing atmospheric pressure afterglow (FAPA) source/direct analysis in real time (DART), dielectric barrier discharge ionization (DBDI)/low-temperature plasma (LTP), desorption electrospray ionization (DESI), and laser desorption (LD) technique. All individual and combined ionization modes can be easily attained by modulating parameters. In particular, the FAPA/DART&DESI mode can realize the detection of polar and nonpolar compounds at the same time with two different ionization mechanisms: proton transfer and charge transfer. The introduction of LD contributes to the mass spectrometry imaging and the surface-assisted laser desorption (SALDI) under ambient condition. Compared with other individual or multi-mode ion source, the iAmIS platform provides the flexibility of choosing different ionization modes, broadens the scope of the analyte detection, and facilitates the analysis of complex samples.

ᅟ
Similar content being viewed by others
Introduction
Since the invention of two most well-known ambient ionization techniques, desorption electrospray ionization (DESI) [1] and direct analysis in real time (DART) [2], ambient mass spectrometry (AMS) [3] has developed over a decade. AMS is currently a hot research area of mass spectrometry owing to its characteristics like fast screening, robustness, and minimal sample preparation. AMS can be divided broadly into two types on the basis of mechanism of desorption and ionization, i.e., plasma-based ion source and electrospray-based ion source [4].
The above two types of ion sources have their exclusive advantages. For plasma-based ion source, originating from the corona discharge atmospheric pressure chemical ionization (APCI) [5, 6], DART, dielectric barrier discharge ionization (DBDI) [7], low-temperature plasma (LTP) [8], and flowing atmospheric pressure afterglow (FAPA) [9, 10] have been reported in succession. Heat-assisting accelerates desorption of analytes and benefits to the rapid detection of substances while it is incapable for the compounds with high molecular weight and thermal labilities [11,12,13,14]. Interestingly, DESI source can directly desorb polar, nonvolatile, and high molecular weight compounds [15,16,17,18] based on the mechanism of solvent extraction [19,20,21], and has become a successful complementary approach of the plasma-based ionization techniques. Similarly, extractive electrospray ionization (EESI) [22], desorption ionization by charge exchange (DICE) [23], nanospray desorption electrospray ionization (nanoDESI) [24, 25], and probe electrospray ionization (PESI) [26] have been proposed and applied successfully. DART electrifies the target compounds with reagent ions of hydrated proton or molecular ion of oxygen generated from the reaction with excited atom (He* or N2*) under high voltage. Normally, the excited atoms are produced by working gas like He, Ne, Ar, or Kr with the energy stored in their excited state decreasing successively [27]. In DART, helium is adopted frequently due to its most effective characteristic of having 19.8 eV energy in its (23S) excited electronic state, which is clearly above the ionization energy of any potentially relevant molecule [2]. The major mechanism of DESI is proton transfer through a “droplet pick-up” process consisting of three consecutive stages: forming thin liquid film, extracting analytes, producing secondary progeny droplets [28, 29]. DESI has its unique advantage in the detection of large molecular weight compounds with low volatility. Besides direct desorption by plasma and electrospray, laser desorption or ablation has also captured the attention of scientists. On demand of detection with high spatial resolution, electrospray-assisted laser desorption ionization (ELDI) [30], laser-induced acoustic desorption/electrospray ionization (LIAD/ESI) [31, 32], laser ablation electrospray ionization (LAESI) [33], and plasma-assisted multiwavelength laser desorption ionization (PAMLDI) [34] were developed subsequently.
Compared to single or multiple mode ambient ionization, combined or hybrid ambient ion sources have been developed to achieve complementary and high-throughput analysis, the reduction of sample complexity, and allowing the simultaneous detection of a broader range of analytes. A simple ambient ionization technique called desorption electrospray/metastable-induced ionization (DEMI) [35] was developed by integrating DESI and DART-type metastable-induced ionization, which could ionize both polar and nonpolar compounds operated in three modes (plasma, spray, or combined mode) offering a versatile platform for ambient MS. However, transferring analytes by a long transfer tube had a low efficiency and resulted in memory effects during sample analysis. Afterwards, a dual electrospray and atmospheric pressure chemical ionization source (ESI/APCI) was raised [36]. Although the same function was implemented like DEMI, the analytes were limited by the range of the DART and DESI. Besides, the helium employed regularly by DART was refused considering the vacuum of mass spectrometer, which narrowed the scope of detectable analytes in DART mode. All above mentioned individual and combined sources are non-cost-effective, waste of space, and application limitation of application of the ambient mass spectrometry.
In this study, we are proposing a platform offering the integrated, comprehensive, cost-effective, and efficient ambient ionization mode. It is integrated ambient ionization source (termed iAmIS) platform, combining the FAPA/DART, DBDI/LTP, and DESI coupled with a continuous wavelength (CW) laser beam to characterize different analytes simultaneously. All individual and combined ionization modes can be easily controlled by modulating parameters, such as DC or AC voltage, and gas flow. Compared with other multimode ionization technique, iAmIS combines five individual ionization modes with the mechanism of proton transfer and charge transfer, which broadens the scope of analytes. This proposed iAmIS platform provides the “all in one” integrated ionization mode, which is promising for analyzing complicated samples under optional ionization mode with diverse ionization mechanism and even targeted analytes with different properties.
Experimental
Reagents
4-Bromobiphenyl (98%) and 4,4′-dibromobiphenyl (97%) were obtained from Beijing Coupling Technology Co., Ltd. (Beijing, China). Ferrocene was purchased from Adamas Reagent Co., Ltd. (Shanghai, China). D-Erythro-sphinganine (SAPH), 1-heptadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine (LPC), and 1,2-dimyristoyl-sn-glycero-3-phosphocholine (PC) were purchased from the Avanti Lipids Polar, Inc. (Alabaster, Alabama). Coconut beautifying oil was purchased from The Body Shop international plc. (UK). Silica gel-coated TLC glass sheets were purchased from Yantai Dexin Biological Technology Co., Ltd. (Shandong, China). The traditional Chinese medicine Ligusticum wallichii and Cinnamomum cassia were purchased from a local pharmacy in China. The perfume was obtained from Calvin Klein Corporation.
Standard Solutions
4-Bromobiphenyl (2 mg/ml), 4,4′-dibromobiphenyl (3 mg/ml), ferrocene (3 mg/ml), SAPH (1 mg/ml), LPC (1 mg/ml), and PC (1 mg/ml) were prepared using MeOH as the solvent.
Design of iAmIS
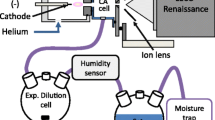
As shown in Figure 1, the whole ion source was composed of electrospray-based source DESI and plasma-based source FAPA. First, a stainless steel needle electrode (i.d. 0.8 mm) was inserted into a quartz glass tube (o.d. 6 mm, i.d. 4 mm) through a double hole of a ferrule. Then the copper foil electrode was wrapped partially on the outer surface of the quartz glass tube with a length of ca. 2.5 cm. The grounding electrode was embedded in the other end of the quartz tube with a distance of 5~7 mm to the needle electrode. When applying a high-voltage DC between the needle electrode and the grounding electrode after the nitrogen or helium was introduced into the quartz tube (discharge voltage: He 400~900 V; N2 1000~3000 V), corona or glow discharge occurred. It was worth noticing that the discharge current can be controlled by changing the value of the external ballast resistance. So, the DART mode could be realized by using a ballast resistance of 1 MΩ with the current lower than 2 mA. The current could be adjusted between 1 and 30 mA after reducing resistance to 5 kΩ, which corresponded to tunable FAPA mode. In addition to being a high-voltage electrode, the needle electrode could also form a pair of electrodes with copper foil electrode to form DBDI/LTP mode. An AC voltage (~ 1.3 kVPP, 19 kHz) was applied to a needle and ring electrode to generate plasma when a nitrogen or helium gas stream flowed through the glass tube with electric field intensity being regulated by adjusting the overlap between the needle and the ring electrode.
In addition to plasma ion source, electrospray-based ionization is another major part introduced to the iAmIS platform. Based on some modifications of the device from Takáts et al. [1], two concentric fused silica capillaries of the DESI (I.D: inner 50 μm, outer 250 μm) were inserted into the quartz glass tube through another hole of ferrule. The tip of the DESI capillary protruded from the quartz capillary up to the length of ca. 2 mm. 0.1% acetic acid in 50% MeOH solution (v/v) was employed at a flow rate of 5 μl/min with the voltage of 4 kV in inner tube as the spray solution, while a flow of nitrogen (0.5 Mpa) was used as the nebulizing gases. All analytes were detected by a Thermo Q Exactive Plus mass spectrometer (Thermo Scientific, USA) with positive ion mode. The capillary temperature was 250 °C and the mass resolution was 70,000.
For direct desorption and ionization, the primary charged species generated by DESI and plasma source were directed onto the sample and deposited on polytetrafluoroethylene (PTFE) plate at an incident angle of 45° for direct desorption and ionization. The distance from the tip of the iAmIS source to the sample surface was ca. 2 mm. The horizontal surface distance between the mass spectrometer inlet and sample was ca. 2 mm. For plasma ion source, the flow rate was ca 3L/min when nitrogen was used as working gas. Because helium is smaller than nitrogen and would break the vacuum of the mass spectrometer because it is easy to enter the entrance of machine. Therefore, it is necessary to turn on the nebulizing gas of DESI to dilute the helium when helium was used as working gas with flow rate ca. 1.2 l/min. When using laser-assisted desorption, the sample was deposited on TLC plate and irradiated by a CW laser beam. The CW laser was operated at 450 nm with a flux energy of ∼ 1 W with the incident angle of 50°. The exit of the iAmIS was 3 mm directly above the sample, and the distance between the exit of the iAmIS source and the MS inlet was 10 mm. Then, desorbed analytes moved upward to join the iAmIS plume for ionization.
Safety Experiment
Electrical insulating gloves were used to prevent electrical shock from the DC and AC high voltages present at iAmIS. Besides, personal protective equipment, such as safety glasses, mask, and laboratory coats, were made essential for each experiment.
Results and Discussion
In spite of the individual ionization mode, compared with the abovementioned triple-mode ionization technique DEMI and dual mode ESI/APCI, combined multiple operation modes are provided in iAmIS with more functions and efficiency. In particular, the FAPA/DART&DESI mode can realize the simultaneous detection of polar and nonpolar compounds. While tunable FAPA mode presents proton transfer and charge transfer ionization mechanisms which can be simply realized by changing the discharge current within a couple of seconds, for the single DART mode, the similar charge transfer mode function cannot be implemented without accessional high-energy heater and sufficient heating time. According to the results of Shelley et al. [37, 38], a relatively low flow rate of working gas with high current is necessary and effective when operating in charge transfer mode, while it was found that proton transfer dominated FAPA ionization with low current and high gas flow rate, corresponding to the DART mode without high temperature. In this mode, the quasi-molecular ions are produced, which indicated that the substances with poor proton affinity were very difficult to be ionized. As a result, the mechanism of charge transfer is noteworthy. As shown in the reactions 1 and 2, the tunable FAPA has two ionization modes of charge transfer and proton transfer, in which M refers to the target molecule. The former can be effectively enhanced with high current and low gas flow rate. By means of the tunable FAPA, a range of compounds that may be ionized is proposed based on the resonance mechanism. Moreover, the helium can be used efficiently with the assistance of nebulizing gas relative to the ESI/APCI source (Figure 4). In addition, the DBDI/LTP&DESI mode was easily carried out by simply changing DC into AC while lower AC voltage is more safe and universal. Since no high temperature was involved, DBDI/LTP&DESI mode could be used in direct detection from skin. In addition to plasma-based and electrospray-based ionization, considering difficulties in the analysis of thick and complicated matrix sample and MS imaging, laser was also introduced in this iAmIS platform.
FAPA/DART&DESI Mode
In this mode, the helium was used as the discharge gas of FAPA/DART. 2.5–5 μl standard methanol solution of 4-bromine biphenyl and SAPH was spotted in pretreated grooves of the PTFE plate, which was designed for reducing the sample diffusion effect on the plate. The plate was placed in front of the mass spectrometer inlet. Sample was desorbed and ionized by the plume generated by FAPA/DART and DESI before it was completely dried. According to the experimental results, only the signal of molecular ion of 4-bromine or protonated ions of SAPH were detected in single FAPA and DESI mode, respectively (Figure 2a, b). In the mixed mode of FAPA&DESI, the signal of 4-bromine biphenyl and SAPH could be detected at the same time (Figure 2c). And interestingly, when using the DART&DESI mode, the signal intensity of 4-bromine biphenyl was less than one order of magnitude of that in FAPA&DESI mode (Figure 2d). It was found that the major ions detected of 4-bromine biphenyl were molecular ions, and the signal intensity of protonated molecular ions was 1/4~1/5 of that of molecular ions. It was shown that the molecular ions were more stable relative to protonated ions.
Furthermore, the possible ionization mechanism was analyzed. Theoretically, when 4-bromine biphenyl lost an electron to form molecular ion, the two intramolecular benzene rings could form resonance structure, which might be more stable than the protonated molecular ions relatively (Figure S1). Therefore, the analyte was more prone to form molecular ion following the charge transfer principle of ion source. Consequently, the analytes with weak proton affinity may be ionized effectively once their molecular ion can form a relatively stable resonance structure. Hence, higher current was applied in FAPA when comparing with DART, and it was 10 and 2 mA in this work. These might induce better results of compounds like 4-bromine biphenyl with charge transfer mechanism. These broaden the scope of analytes compared with other multimode ionization techniques. In addition, the high temperature generated by the large current of FAPA contributed to better signal during the analysis process, too.
A traditional Chinese medicine Ligusticum wallichii was used to test the practicability of FAPA & DESI mode of iAmIS. The experimental conditions were similar to those mentioned above besides a higher flow rate of 30 μl/min of spray solution. Solid Ligusticum wallichii was put under the iAmIS directly in experiment. As shown in Figure 3, the relatively strong peaks in ion clusters detected in DESI mode and FAPA (10 mA) mode were labeled with black star and black circle, respectively. Ions were detected at m/z 191.1057, 208.1322, 236.1632, 323.0560, 337.0350, 468.9859, and 483.1448 in single DESI mode. In FAPA (10 mA) mode, ions at m/z 191.1056, 208.1323, 385.2359, and 405.2814 were found. Totally, the above ions were all existed in FAPA (10 mA) & DESI mode. With the same conditions, the Cinnamomum cassia and perfume were also tested (Figure S2, S3).
It is noteworthy that, to ensure vacuum formation, helium was not applied for the ionization of 4-bromine biphenyl at the beginning. There was no obvious signal of 4-bromine biphenyl when nitrogen was used, which suggested that the mechanism of charge transfer was more likely to occur when helium was used as working gas. Fortunately, it was accidentally found that the vacuum was not decreased fleetly when using helium. The possible reason is that after mixing with the high flow rate nebulizing gas nitrogen, the movement of smaller and lighter He was disturbed. Little He will finally enter MS. However, the desorbed substance will react with the O2+· created from the reaction with exited He to produce M+· for further detection by MS (Figure 4). Then a more effective and functional iAmIS can be realized by the “diluted” helium with less influence on vacuum.
DBDI/LTP&DESI Mode
In addition to the FAPA/DART&DESI mode, many other combined modes can be provided by iAmIS platform. By simply changing DC to AC, the DBDI/LTP&DESI mode was realized. Since DBDI/LTP&DESI mode does not involve high-temperature operation, so it can contact the skin directly. Standard methanol solutions of ferrocene and SAPH were chosen to test the reliability of the mode. The results were just similar to the FAPA/DART&DESI mode, where only ferrocene was detected in DBDI/LTP mode (Figure 5a) and SAPH was the only response compound in DESI mode (Figure 5b), while in the DBDI/LTP&DESI mode, the mixture signals could be detected simultaneously (Figure 5c). Compared with the experimental result in LD-ESI + APCI mass spectra from Cheng et al. [36], the dominated peak of ferrocene is the ferrocene radical ions at m/z 186 while the AC voltage (4.3–8.3 kVPP, 19 kHz) was conducted. In our experiment, the DBDI/LTP mode was realized by applying an AC voltage (~ 1.3 kVPP, 19 kHz). The major peaks of ferrocene in our work are the ferrocene radical ions at m/z 186 and the protonated ferrocene ions at m/z 187. The actual structure for these two sources and the AC frequency are all the same. The only difference of voltage might be the major reason for this phenomenon. It is believed that, similar to tunable FAPA, the lower AC voltage has two kinds of ionization mechanisms, which is of universality and safety.
According to the above results, all compounds analyzed with different ionization sources are summarized in Table 1. It is obvious that multifarious ion sources offer different possibilities when analyzing various molecules. Substance being detected usually undergoes two processes: desorption and ionization. From the point of proton affinity, it is easy to hydrogenate on the molecule like lipid. However, they are quite hard to be desorbed. From our experiment, many lipids have been tried for DART-MS analysis while few of them can be detected. Besides, high temperature is necessary to ensure the good results of detection considering desorption. In a word, the desorption and ionization should be considered synchronously during the detection of different substances using iAmIS. The proposed mechanisms of different ionization modes, based on the ionization capacity (without considering desorption) in iAmIS, are listed in Table 2 that can be used for the guidance while choosing the ionization mode.
LD-FAPA&DESI Mode
Besides the abovementioned plasma-based and electrospray-based desorption and ionization modes, laser is another alternative desorption mode in iAmIS. Considering difficulties in the analysis of thick and complicated matrix sample and MS imaging, laser was introduced in this iAmIS platform. The mass spectrometer can be effectively protected from the contamination using laser desorption when analyzing actual complex samples, such as oil and blood. In addition, the lasers are key part in the area of ambient mass spectrometry imaging. Therefore, the introduction of laser was necessary for the versatility of iAmIS. High-energy solid-state laser and simple CW laser can be used in this platform; the former provides high spatial resolution and the latter is cost-effective and practical. Herein, a simple CW laser was used to identify the operability of LD mode of iAmIS. The coconut essential oil was spotted on the TLC plate for drying before desorption. Then, the sample was desorbed by laser, ionized by interaction with the ion plume ejected by the iAmIS placed horizontally and subsequently analyzed by mass spectrometer (Figure S7). Nitrogen was chosen as discharge gas with a DC current of 3.5 mA. Figure 6 shows the results of laser desorption of coconut oil in the LD-DESI mode, LD-FAPA mode, and LD-FAPA&DESI mode. In LD-DESI mode, ions were detected at m/z 304.2844, 327.2524, 344.2793, 355.2838, 372.3107, 411.3469, 429.3572, 443.3730, 460.3993, 469.3886, and 488.3944 (Figure 6a). In LD-FAPA mode, ions were detected at m/z 304.2844, 316.2846, 335.2662, 344.2792, 372.3106, 443.3731, 460.3992, 469.3890, and 488.3944 (Figure 6b). In LD-FAPA&DESI mode, ions detected in either LD-FAPA or LD-DESI modes were both detected (Figure 6c).
Conclusion
In this study, a versatile integrated ambient ionization source platform was developed integrating the FAPA/DART, DBDI/LTP, and DESI as well as CW laser. The optimum ionization mode can be chosen in terms of different mechanisms while using this iAmIS platform. Hence, by adjusting the experimental parameters, the FAPA/DART&DESI mode can ionize polar and nonpolar compounds at the same time with two different ionization mechanisms: proton transfer and charge transfer. The iAmIS platform adds a new dimension to ambient MS for potential ionization of electron-rich compounds with a relatively weak proton affinity, increasing the range and number of detectable analytes. Additionally, the performance of iAmIS can be more effective by using helium after “dilution” by the nebulizing gas, to maintain the vacuum. Incorporation of LD technology into iAmIS also made an extension in the area of ambient mass spectrometry imaging. As a result, the more comprehensive functions of iAmIS are proposed for analysis of complicated samples containing compounds with varying properties.
References
Takáts, Z., Wiseman, J.M., Gologan, B., Cooks, R.G.: Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 306, 471–473 (2004)
Cody, R.B., Laramee, J.A., Durst, H.D.: Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 77, 2297–2302 (2005)
Cooks, R.G., Ouyang, Z., Takats, Z., Wiseman, J.M.: Ambient mass spectrometry. Science. 311, 1566–1570 (2006)
Monge, M.E., Harris, G.A., Dwivedi, P., Fernández, F.M.: Mass spectrometry: recent advances in direct open air surface sampling/ionization. Chem. Rev. 113, 2269–2308 (2013)
Horning, E., Horning, M., Carroll, D., Dzidic, I., Stillwell, R.: New picogram detection system based on a mass spectrometer with an external ionization source at atmospheric pressure. Anal. Chem. 45, 936–943 (1973)
Dzidic, I., Carroll, D., Stillwell, R., Horning, E.: Comparison of positive ions formed in nickel-63 and corona discharge ion sources using nitrogen, argon, isobutane, ammonia and nitric oxide as reagents in atmospheric pressure ionization mass spectrometry. Anal. Chem. 48, 1763–1768 (1976)
Na, N., Zhao, M., Zhang, S., Yang, C., Zhang, X.: Development of a dielectric barrier discharge ion source for ambient mass spectrometry. J. Am. Soc. Mass Spectrom. 18, 1859–1862 (2007)
Harper, J.D., Charipar, N.A., Mulligan, C.C., Zhang, X., Cooks, R.G., Ouyang, Z.: Low-temperature plasma probe for ambient desorption ionization. Anal. Chem. 80, 9097–9104 (2008)
Shelley, J.T., Chan, G.C.-Y., Hieftje, G.M.: Understanding the flowing atmospheric-pressure afterglow (FAPA) ambient ionization source through optical means. J. Am. Soc. Mass Spectrom. 23, 407–417 (2012)
Shelley, J.T., Wiley, J.S., Hieftje, G.M.: Ultrasensitive ambient mass spectrometric analysis with a pin-to-capillary flowing atmospheric-pressure afterglow source. Anal. Chem. 83, 5741–5748 (2011)
Cody, R.B.: Observation of molecular ions and analysis of nonpolar compounds with the direct analysis in real time ion source. Anal. Chem. 81, 1101–1107 (2009)
Zhou, M., McDonald, J.F., Fernández, F.M.: Optimization of a direct analysis in real time/time-of-flight mass spectrometry method for rapid serum metabolomic fingerprinting. J. Am. Soc. Mass Spectrom. 21, 68–75 (2010)
Edison, S., Lin, L.A., Gamble, B.M., Wong, J., Zhang, K.: Surface swabbing technique for the rapid screening for pesticides using ambient pressure desorption ionization with high-resolution mass spectrometry. Rapid Commun. Mass Spectrom. 25, 127–139 (2011)
Forbes, T.P., Sisco, E.: Trace detection and competitive ionization of erythritol tetranitrate in mixtures using direct analysis in real time mass spectrometry. Anal. Methods. 7, 3632–3636 (2015)
Bagatela, B.S., Lopes, A.P., Cabral, E.C., Perazzo, F.F., Ifa, D.R.: High-performance thin-layer chromatography/desorption electrospray ionization mass spectrometry imaging of the crude extract from the peels of Citrus aurantium L. (Rutaceae). Rapid Commun. Mass Spectrom. 29, 1530–1534 (2015)
Przybylski, C., Gonnet, F., Buchmann, W., Daniel, R.: Critical parameters for the analysis of anionic oligosaccharides by desorption electrospray ionization mass spectrometry. J. Mass Spectrom. 47, 1047–1058 (2012)
Suni, N.M., Aalto, H., Kauppila, T.J., Kotiaho, T., Kostiainen, R.: Analysis of lipids with desorption atmospheric pressure photoionization-mass spectrometry (DAPPI-MS) and desorption electrospray ionization-mass spectrometry (DESI-MS). J. Mass Spectrom. 47, 611–619 (2012)
Eckert, P.A., Roach, P.J., Laskin, A., Laskin, J.: Chemical characterization of crude petroleum using nanospray desorption electrospray ionization coupled with high-resolution mass spectrometry. Anal. Chem. 84, 1517–1525 (2012)
Green, F., Salter, T., Gilmore, I., Stokes, P., O'Connor, G.: The effect of electrospray solvent composition on desorption electrospray ionisation (DESI) efficiency and spatial resolution. Analyst. 135, 731–737 (2010)
Badu-Tawiah, A., Cooks, R.G.: Enhanced ion signals in desorption electrospray ionization using surfactant spray solutions. J. Am. Soc. Mass Spectrom. 21, 1423–1431 (2010)
Badu-Tawiah, A., Bland, C., Campbell, D.I., Cooks, R.G.: Non-aqueous spray solvents and solubility effects in desorption electrospray ionization. J. Am. Soc. Mass Spectrom. 21, 572–579 (2010)
Chen, H., Venter, A., Cooks, R.G.: Extractive electrospray ionization for direct analysis of undiluted urine, milk and other complex mixtures without sample preparation. Chem. Commun. 2042–2044 (2006)
Chan, C.-C., Bolgar, M.S., Miller, S.A., Attygalle, A.B.: Desorption ionization by charge exchange (DICE) for sample analysis under ambient conditions by mass spectrometry. J. Am. Soc. Mass Spectrom. 21, 1554–1560 (2010)
Roach, P.J., Laskin, J., Laskin, A.: Nanospray desorption electrospray ionization: an ambient method for liquid-extraction surface sampling in mass spectrometry. Analyst. 135, 2233–2236 (2010)
Roach, P.J., Laskin, J., Laskin, A.: Molecular characterization of organic aerosols using nanospray-desorption/electrospray ionization-mass spectrometry. Anal. Chem. 82, 7979–7986 (2010)
Hiraoka, K., Nishidate, K., Mori, K., Asakawa, D., Suzuki, S.: Development of probe electrospray using a solid needle. Rapid Commun. Mass Spectrom. 21, 3139–3144 (2007)
Hiraoka, K., Ninomiya, S., Chen, L.C., Iwama, T., Mandal, M.K., Suzuki, H., Ariyada, O., Furuya, H., Takekawa, K.: Development of double cylindrical dielectric barrier discharge ion source. Analyst. 136, 1210–1215 (2011)
Costa, A.B., Cooks, R.G.: Simulation of atmospheric transport and droplet–thin film collisions in desorption electrospray ionization. Chem. Commun. 3915–3917 (2007)
Gao, L., Li, G., Cyriac, J., Nie, Z., Cooks, R.G.: Imaging of surface charge and the mechanism of desorption electrospray ionization mass spectrometry. J. Phys. Chem. C. 114, 5331–5337 (2010)
Lin, S.-Y., Huang, M.-Z., Chang, H.-C., Shiea, J.: Using electrospray-assisted laser desorption/ionization mass spectrometry to characterize organic compounds separated on thin-layer chromatography plates. Anal. Chem. 79, 8789–8795 (2007)
Cheng, S.-C., Cheng, T.-L., Chang, H.-C., Shiea, J.: Using laser-induced acoustic desorption/electrospray ionization mass spectrometry to characterize small organic and large biological compounds in the solid state and in solution under ambient conditions. Anal. Chem. 81, 868–874 (2009)
Cheng, S.-C., Huang, M.-Z., Shiea, J.: Thin-layer chromatography/laser-induced acoustic desorption/electrospray ionization mass spectrometry. Anal. Chem. 81, 9274–9281 (2009)
Nemes, P., Vertes, A.: Laser ablation electrospray ionization for atmospheric pressure, in vivo, and imaging mass spectrometry. Anal. Chem. 79, 8098–8106 (2007)
Zhang, J., Zhou, Z., Yang, J., Zhang, W., Bai, Y., Liu, H.: Thin layer chromatography/plasma assisted multiwavelength laser desorption ionization mass spectrometry for facile separation and selective identification of low molecular weight compounds. Anal. Chem. 84, 1496–1503 (2012)
Nyadong, L., Galhena, A.S., Fernández, F.M.: Desorption electrospray/metastable-induced ionization: a flexible multimode ambient ion generation technique. Anal. Chem. 81, 7788–7794 (2009)
Cheng, S.-C., Jhang, S.-S., Huang, M.-Z., Shiea, J.: Simultaneous detection of polar and nonpolar compounds by ambient mass spectrometry with a dual electrospray and atmospheric pressure chemical ionization source. Anal. Chem. 87, 1743–1748 (2015)
Shelley, J.T., Wiley, J.S., Chan, G.C., Schilling, G.D., Ray, S.J., Hieftje, G.M.: Characterization of direct-current atmospheric-pressure discharges useful for ambient desorption/ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 20, 837–844 (2009)
Badal, S.P., Michalak, S.D., Chan, G.C.-Y., You, Y., Shelley, J.T.: Tunable ionization modes of a flowing atmospheric-pressure afterglow (FAPA) ambient ionization source. Anal. Chem. 88, 3494–3503 (2016)
Funding
This work was financially supported by the Chinese Ministry of Science and Technology, the National Instrumentation Program (2016YFF0100303), and the National Natural Science Foundation of China (Grant Nos. 21527809, 21405006).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOCX 1762 kb)
Rights and permissions
About this article
Cite this article
Ai, W., Nie, H., Song, S. et al. A Versatile Integrated Ambient Ionization Source Platform. J. Am. Soc. Mass Spectrom. 29, 1408–1415 (2018). https://doi.org/10.1007/s13361-018-1949-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-018-1949-3