Abstract
Nitrogen can be an inexpensive alternative to helium used by direct analysis in real time (DART), especially in consideration of the looming helium shortage. Therefore, the ionization mechanism of positive-ion N2 DART has been systematically investigated. Our experiments suggest that a range of metastable nitrogen species with a variety of internal energies existed and all of them were less energetic than metastable helium atoms. However, compounds with ionization energies (IE) equal to or lower than 10.2 eV (all organic compounds except the extremely small ones) can be efficiently ionized. Because N2 DART was unable to efficiently ionize ambient moisture and common organic solvents such as methanol and acetonitrile, the most important ionization mechanism was direct Penning ionization followed by self-protonation of polar compounds generating [M+H]+ ions. On the other hand, N2 DART was able to efficiently ionize ammonia, which was beneficial in the ionization of hydrogen-bonding compounds with proton affinities (PA) weaker than ammonia generating [M+NH4]+ ions and large PAHs generating [M+H]+ ions through proton transfer. N2 DART was also able to efficiently ionize NO, which led to the ionization of nonpolar compounds such as alkanes and small aromatics generating [M–(2m+1)H]+ (m=0,1…) ions. Lastly, metastable nitrogen species was also able to produce oxygen atoms, which resulted in increased oxygen adducts as the polarity of organic compounds decreased. In comparison with He DART, N2 DART was approximately one order of magnitude less sensitive in generating [M+H]+ ions, but could be more sensitive in generating [M+NH4]+ ions.

ᅟ
Similar content being viewed by others
Introduction
Direct analysis in real time mass spectrometry (DART-MS) enables rapid and noncontact analysis of samples in forms of gas, liquid, and solid at ambient condition with little sample preparation and without chromatographic separation. Since its introduction in 2005 [1], DART-MS has been progressed into an established analytical technique for rapid identification of numerous types of samples [2,3,4,5,6,7,8,9,10]. It has been utilized in biological studies, chemical reaction monitoring, clinical measurements, environmental analysis, food quality testing, forensic drug analysis, medical diagnostics, pharmaceutical analysis, and warfare agent detection [2,3,4,5,6,7,8,9]. Specifically, the determination of drugs and drug-like compounds in different samples with DART-MS was summarized by Chernetsova and Morlock in 2011 [2]. Forensic drug analysis with DART-MS was reviewed by Lesiak and Shepard in 2014 [7]. The entire set of applications of DART-MS were described by Gross in 2014 [6]. More recent applications of DART-MS in bioanalytical sciences can be found in a review article published by Smoluch et al. in 2016 [9].
In order to deal with a wide variety of samples including different sizes and shapes, multiple configurations of the DART ion source have been developed [6, 10, 11]. The original DART ion source, i.e., DART-100, was designed for the JEOL AccuTOF mass spectrometer to be directly mounted in front of its orifice. However, the coupling of a DART ion source with other brands of mass spectrometers, the so-called Vapur interface, an additional compact membrane pumping stage, was used to avoid compromise of vacuum by DART discharge gas flowing directly into the orifice of the mass spectrometers. The recent DART ion source, i.e., DART-SVP (simplified voltage and pressure model), comes with a redesigned DART controller to fix the gas flow rate and pressure in order to improve lab-to-lab reproducibility. The DART-SVP also comes with an optional surface desorption mode and a transmission mode. The surface desorption mode is well suited for the analysis of tablets, leaves, clothes, planar plates of high performance thin layer chromatography (HPTLC), and melting point capillaries carrying liquid, powder, or deposited film. In this mode, the DART-SVP can work at an angle up to 45° relative to the sampling surface. The transmission mode is achieved with a porous surface, e.g., mosquito bed-netting [12], foam swabs [13], and wire mesh [11], where samples are either collected or deposited. With this mode, good quantitative results have been reported for the analysis of select few fungicides and carbendazim, in a variety of orange juice products [11].
Despite multiple configurations, the inside of the DART ion source has remained basically the same. It consists of two chambers through which the DART discharge gas flows [1, 10]. In the first region, the DART discharge gas is exposed to a DC point-to-plane glow discharge, generating a plasma containing a variety of transient highly energetic species such as ions, electrons, and metastable species. In the second region, a gas heater permits heating of the DART discharge gas. The exit electrode is biased to a positive potential for operation in positive-ion mode or to a negative potential for operation in negative-ion mode. At the exit, a ceramic insulator cap is used to protect the operator from accidental contact with the exit electrode. However, there is a minor difference between DART-100 and DART-SVP. The intermediate electrode in the original DART-100 was thought to remove ions and electrons from the DART gas stream, but this electrode was determined to be unnecessary and removed from later generations of the DART ion source. Nevertheless, it is believed that both DART-100 and DART-SVP prevent the majority of ionic species from exiting the ion source and reaching the ionization region by plasma-ion filtering through negative bias applied to the discharge needle and various electrodes [1, 5, 6, 10].
So far, helium has been predominantly utilized as the DART discharge gas in numerous DART-MS applications. The ionization mechanism of He DART has been described in great detail in a few publications [1, 14,15,16,17]. It is generally recognized that the ionization mechanism of He DART is closely related to the gas phase ion-molecule reactions in atmospheric pressure chemical ionization (APCI). However, it differs from other forms of APCI in the initial ion formation step, i.e., Penning ionization. The electric glow discharge of helium primarily produces long-lived (“metastable”) helium 23S electronic excited state atoms with an internal energy of 19.8 eV, higher than any other metastable species, and an extremely long predicted unimolecular lifetime of approximately 8000 s [18]. Consequently, metastable helium atoms are able to ionize all components of ambient gases such as nitrogen, oxygen, ambient moisture, and ammonia with extremely high efficiency to generate reagent ions. Following the initial Penning ionization, a cascade of gas-phase ion-molecule reactions proceed in the vicinity of the sample surface subject to analysis, which virtually ionize all organic compounds due to their lower ionization energy (IE) than metastable helium atoms and/or stronger proton affinity (PA) than protonated water clusters, generating [M+H]+, M+• , [M−H]+, and/or [M+NH4]+ ions in the positive-ion mode.
Because nitrogen is inexpensive and readily available, it has been used in all the configurations of DART ion source to replace helium when they are put in standby mode for improved cost efficiency. However, nitrogen has been only used as the DART discharge gas occasionally [19,20,21,22,23,24,25]. The inventors of DART did suggest nitrogen as an alternative to helium as the DART discharge gas, but little data was published to promote the application of N2 DART [1, 14]. Theoretically, N2 DART should have lower ionization efficiency, but less in-source fragmentation than He DART because internal energy of metastable helium atoms is the highest among all possible metastable species. This has been confirmed by three comparative studies by Borges et al. [19], Nah et al. [23], and Jorabchi et al. [24]. Additionally, a more recent article by Dumlao et al. [26] reported that the use of He DART resulted in significantly more internal energy deposition on benzylammonium thermometer ions than the use of dielectric barrier discharge ionization (DBDI). On the other hand, N2 DART has also been successfully used in applications where He DART was inappropriate by Fernandez and coworkers [20,21,22]. On one occasion, DART was successfully coupled with a drift tube ion mobility spectrometer (DTIMS) in which the instrument used nitrogen as both the DART discharge gas and DTIMS drift gas, allowing for a high electric field to be used for ion separation while keeping cost-of-use low [20, 22]. On another occasion, a proof-of-principle electro-thermal vaporizer was coupled to DART-MS for water contaminant analysis during space missions in which the instrument used nitrogen as the DART discharge gas because neither the low molecular weight of the analytes (30 to 94 Da) nor the gas availability constraints on board the International Space Station (ISS) permits the use of helium as the DART discharge gas.
At present, the ionization mechanism of N2 DART has not been systematically studied. In consideration of the looming helium shortage over the past 10 years [27], N2 DART is especially attractive. It is therefore anticipated that the utilization of N2 DART may become widely used by a systematic investigation of its ionization mechanism.
Experimental
Materials and Reagents
All solvents, including acetonitrile, methanol, ethanol, 2-propanol, ethyl acetate, acetone, tetrahydrofuran (THF), N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), methylene chloride, cyclohexane, hexanes, heptane, isooctane, toluene, and chlorobenzene were either HPLC grade or Certified A.C.S. grade, purchased from Fisher Scientific (Pittsburgh, PA, USA). Most of the other organic compounds, including phenethylamine, benzophenone, diethyl phthalate, metolachlor, progesterone, reserpine, propylamine, isopropalamine, tert-butylamine, acetaldehyde, D-(+)-glucose, glyceryl trioctanoate, n-hexadecane, anisole, naphthalene, fluorene, anthracene, 9-methylanthracene, pyrene, benzo(a)pyrene, and dibenzo(ah)anthracene were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sulfometuron methyl ester was purchased from AK Scientific Inc. (Union City, CA, USA). PEG 200 was purchased from Alfa Aesar (Ward Hill, MA, USA). High purity/high pressure helium (minimum purity 99.997%), high purity/high pressure nitrogen (minimum purity 99.998%), and BIP technology nitrogen (minimum purity 99.9999%) were purchased from Airgas Inc. (Radnor Township, PA, USA). A comparison study between high purity/high pressure and BIP technology nitrogen did not show any difference in N2 DART-MS analysis.
Instrument and Method
All experiments were performed using a JEOL (Peabody, MA, USA) JMS-T100LC (AccuTOF) orthogonal time-of-flight (TOF) mass spectrometer with an IonSense (Danvers, MA, USA) DART-100 ion source. The distance between the outlet of the DART discharge gas and the inlet of the orifice 1 of the AccuTOF was about 1 cm.
For the analysis of solvents and similar volatile organic compounds, including anisole, acetaldehyde, and n-hexadecane by both N2 and He DART-MS, sampling was easily achieved by dipping the closed end of a melting point capillary into the neat compound so that a droplet of the sample (~1 μL) adhered to the end of the melting point capillary. Afterwards, sample introduction was accomplished by moving the closed end of the melting point capillary across the gas stream between the DART ion source and the orifice 1 of the AccuTOF. Propylamine, isopropylamine, and tert-butylamine were analyzed similarly, but with their 1% (v/v) solution in methylene chloride. All other less volatile compounds were analyzed as 10 μg/mL solution in methylene chloride, methanol, or acetonitrile by N2 DART-MS or as 1 μg/mL solution in methylene chloride, methanol, or acetonitrile by He DART-MS. In addition, between sampling and sample introduction, the droplet from a solution at the closed end of the melting point capillary was air-dried for approximately 1 min for methylene chloride or 2 min for methanol and acetonitrile as the solvent. However, this air-drying process was unnecessary for calibration with the 10% (v/v) PEG 200 solution in methylene chloride.
The AccuTOF parameters were tuned to a resolving power of 6000 (full width at half maximum) with a 100 μg/mL reserpine solution in methanol using electrospray ionization (ESI). The parameters of the DART-100 ion source and the interface between the DART-100 ion source and the AccuTOF were optimized by analyzing the 100 μg/mL reserpine solution in methanol with He DART-MS to achieve the best signal. Detailed settings of the parameters of the DART ion source and the interface between the DART ion source and the AccuTOF were as follows: gas flow control for He DART-MS. 3.5; gas flow control for N2 DART-MS, 5; gas temperature control, 300 oC; needle voltage, 3500 V; electrode #1 voltage, 250 V; electrode #2 voltage, 60 V; orifice 2 voltage, 3 V; ring lens voltage, 10 V; and orifice 1 temperature, 100 oC. It is noted that N2 DART-MS used the maximum gas flow allowed by the DART controller. In addition, the orifice 1 voltage was varied between 5 to 150 V because it had a significant effect on the ionization products. Finally, the mass acquisition range was m/z 10–400 and the spectra recording interval was 0.5 s for all samples.
Calibration was accomplished with the 10% (v/v) PEG 200 solution in methylene chloride. Its ionization products by N2 DART-MS were examined carefully at different orifice 1 voltages, i.e., 5, 10, 30, 50, 80, 100, 120, and 150 V. At low orifice 1 voltages, [M+NH4]+ ions were predominant. As the orifice 1 voltage increased, [M+H]+ and then [M+H−H2O]+ ions were predominant. At very high orifice 1 voltages, the fragmental [CH2O+H]+ ion of PEG 200 and the [M−H]+ and [M−Cl] + ions of methylene chloride were observed. Therefore, accurate calibration was able to be performed down to m/z 31. The ions generated, their elemental composition, and theoretically calculated monoisotopic masses are summarized in Supplementary Table S1.
.
Results and Discussion
Ionization of Ambient Gases
Cody et al. [1] indicated that N2 DART primarily produces vibronically excited state nitrogen molecules. The lowest metastable state of N2 is at 6.2 eV, not high enough to ionize most molecules [28]. Although there are higher electronic and vibrational states, up to the E3Σg+state at 11.88 eV, they are dissociative at even moderate vibronic levels [28] and short-lived in comparison with the helium 23S electronic excited state atoms, approximately 10 μs [29]. It is known that both ground state N(4S) atoms and metastable N(2D) and N(2P) atoms, 2.38 and 3.58 eV up, respectively, from the ground state atoms, are produced in N2 discharges [30, 31]. These may recombine to produce a range of excited N2 in states with at least 9.8 eV and up to approximately 12.3 eV in excess energy. The ionization energies (IE) of ambient gases, including nitrogen, water, oxygen, ammonia, and nitric oxide are 15.6, 12.6, 12.1, 10.0, and 9.3 eV, respectively [32]. Consequently, water cluster ions may not be in the background mass spectra of N2 DART-MS, but formation of O2+, NH4+, and NO+ ions may be possible. It is noted that water cluster ions, NH4+ and NO+ are commonly observed in the background mass spectra of He DART-MS, simply because metastable helium atoms have an internal energy of 19.8 eV [1, 14]. However, the O2+ ion was only reported in atypical conditions [1, 14].
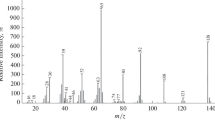
Our experiments showed noteworthy differences between N2 and He DART. First, the background mass spectra of N2 DART-MS were typically two orders of magnitude weaker in ion intensity than helium DART. Secondly, most of the ions observed varied in mass from day to day. Our identification of the ions originated from the ambient gases in the background mass spectra of N2 DART-MS was achieved after a careful examination of the mass spectra of the calibrant, i.e., the PEG 200 10% (v/v) solution in methylene chloride, and a total understanding of its ionization products at different orifice 1 voltages, i.e., 5, 10, 30, 50, 80, 100, 120, and 150 V. Figure 1 shows the mass spectra of the calibrant and the background at orifice 1 voltage of 120, 30, and 5 V.
The calibration started at 120 V orifice 1 voltage because the identifiable ion from the calibrant had the lowest m/z value, i.e., 31.0184 (Figure 1a). Subsequently, O2+ and NO+ ions were identified with 0.8 and 0.0 mDa mass error (Supplementary Table S2), respectively, because their m/z values were very close or within the calibration range (Figure 1b). The [H2O+H]+ ion was identified with 5.2 mDa mass error (Supplementary Table S2) because its m/z value , i.e., 19.0184, was approximately 12 Da lower than the calibration range (Figure 1b). The calibration proceeded at 30 V orifice 1 voltage and the identifiable ion from the calibrant had the lowest m/z value at 45.0340 (Figure 1c). The ion at m/z 17.9960 in Figure 1d was identified as NH4+with 38.4 mDa mass error (Supplementary Table S2) because its m/z value was approximately 27 Da lower than the calibration range (Figure 1d). It was not identified as H2O+• because of the identification of the [H2O+H]+ ion in Figure 1b, in addition to the identification of the [HO(CH2CH2O)3H+NH4]+ ion in Figure 1c with 0.2 mDa mass error. Finally, the calibration range at 5 V orifice 1 voltage was down to 18.0344 due to the identified NH4+ ion (Figure 1e). Subsequently, [NH3+NH4]+ and [H2O+NH4]+ ions were identified with 0.4 and 0.9 mDa mass errors, respectively (Figure 1f and Supplementary Table S2). It is noted that previously peaks at 18.02 and 36.03 Da were identified as H2O+• and 2H2O+• at the background mass spectrum of N2 DART-MS, but mass errors of up to 10 mDa were observed for m/z values lower than 150 Da [21]. We believe that these ions are theoretically unlikely for N2 DART-MS due to their loss via self-protonation reaction. Other ions identified in the background mass spectra of N2 DART-MS included hydrocarbon ions at high orifice 1 voltage and their N and/or O adducts at low orifice 1 voltage, which was consistent with the results obtained in the analysis of nonpolar solvents as described later.
The background mass spectra of He DART-MS was acquired under identical conditions (Supplementary Figure S1). The same process of mass calibration and ion identification was also performed with mass errors listed in Supplementary Table S3. While the identification of [NH3+H]+, [H2O+H]+, [CH3OH+H]+, [2H2O+H]+, [CH3CN+H]+, and [CH3COCH3+H]+ at 5 and 30 V orifice 1 voltage was not a surprise (Supplementary Figure S1a and S1b) [14], the identification of highly abundant O2+ and NO+ ions at 120 V orifice 1 voltage was interesting (Supplementary Figure S1c). It is noted that these ions were unlikely to be mainly generated by in-source fragmentation of other ions at lower orifice voltage such as the NH4+, [H2O+H]+, [CH3OH+H]+, [2H2O+H]+, [CH3CN+H]+, [CH3COCH3+H]+ because with N2 DART-MS the abundance of [CH3OH+H]+, [CH3CN+H]+, and [CH3CH2OH+H]+ ions was also much higher at orifice 1 voltage of 120 V than 30 V (Figure 2).
Ionization of Polar Solvents
Although [H2O+H]+, O2+, NO+, and NH4+ ions were identified in the background mass spectra of N2 DART-MS, their intensities were low. In order to further test the ionization ability of N2 DART, polar solvents, including acetonitrile, methanol, ethanol, 2-propanol, ethyl acetate, acetone, THF, DMF and DMSO, were analyzed. Their mass spectra at orifice 1 voltages of 5, 10, 30, 50, 80, 100, 120, and 150 V were acquired and carefully examined. It was found that at 5 V orifice 1 voltage, [M+NH4]+ ions were predominant for all the polar solvents except DMSO and DMF (Figure 2a). As the orifice 1 voltage increased to 30 V, [2M+H]+ and/or [M+H]+ ions became predominant (Figure 2b). Highly abundant [2M+H]+ and/or [M+H]+ ions were even observed at very high orifice 1 voltages, e.g., 120 V (Figure 2c). Water loss from the [2M+H]+ ions by methanol, ethanol, 2-propanol, and THF were also observed at higher orifice 1 voltages. The mass spectra of THF and 2-propanol were much more complicated by fragment ions than those of other polar solvents. The hydride abstraction ions of THF (Supplementary Figure S2), which were observed not only at 120 and 30, i.e., [M+H-H2]+, but also at 5 V orifice 1 voltage, i.e., [M+NH4-H2]+, was interesting; and the corresponding reaction that led to its formation will be described later. It is noted that all the ions identified were within ±2 mDa mass error (Supplementary Table S4).
Generation of [M+H]+ Ions
In Figure 2b and c, the polar solvents along the x-axis, i.e., acetonitrile, methanol, ethanol, 2-propanol, ethyl acetate, acetone, THF, DMSO, and DMF, are ordered by decreasing IEs, i.e., 12.2, 10.8, 10.5, 10.2, 10.0, 9.7, 9.4, 9.1, and 9.1 eV, respectively. Therefore, Figure 2b and c indicate that metastable nitrogen species of N2 DART were able to efficiently ionize compounds with IEs of 10.2 eV (IE of 2-propanol) and lower. While the metastable nitrogen species of N2 DART were also able to ionize compounds with IEs higher than 10.2 eV, up to 12.6 eV (IE of water), the intensities of the resultant ions were much lower, indicating a much lower population of the metastable nitrogen species with internal energy higher than 10.2 eV. It is noted that the low abundant NH4+ and NO+ ions in the background mass spectra of N2 DART-MS were probably due to low concentration of their corresponding neutrals in the source, even though the IEs of NH3 and NO are 10.0 and 9.3 eV, respectively [17].
Because all organic compounds, except for the extremely small ones such as methane, methanol and acetonitrile, have IEs lower than 10.2 eV, it is concluded that they should be efficiently ionized by N2 DART. However, for best sensitivity it is believed that they should be analyzed in solid form because N2 DART is unable to ionize common solvents such as water, methanol, and acetonitrile. In our experiments, the solid state was achieved by simply preparing them in a solution with a solvent, sampling approximately 1 μL by dipping the closed end of a melting point capillary into the solution, then air-drying for approximately 1 min for methylene chloride as the solvent or 2 min for methanol and acetonitrile as the solvent before DART ionization.
A group of representative polar compounds, including phenethylamine, benzophenone, diethyl phthalate, metolachlor, progesterone, and sulfometuron methyl ester, which are significant in either forensic, food, drug, or environmental analysis, and also possess different functional groups such as amine, amide, ketone, and ester, was subsequently selected to estimate the limit of detection (LOD) of N2 versus He DART-MS. They were prepared at 0.1, 1, 10, and 100 μg/mL concentration. The LOD was estimated to be the lowest concentration when the experimentally measured monoisotopic mass was within ±2 mDa of the theoretically calculated monoisotopic mass of all the representative polar compounds with a minimum of triplicate measurements (Supplementary Table S5). The LOD of N2 and He DART-MS was estimated to be approximately 10 and 1 μg/mL, respectively. Figure 3 and Supplementary Figure S3 illustrate N2 and He DART-MS spectra of the representative polar compounds, respectively. The oxygen adduct ions of N2 DART-MS were interesting (Figure 3a and e); and the reaction that led to their formation will be described later. In addition, it can be seen that N2 DART caused less in-source fragmentation of diethyl phthalate and progesterone than He DART (Supplementary Figure S3c and S3e), which can be attributed to the lower internal energy of metastable nitrogen species than helium atoms.
N2 DART-MS spectra of: (a) phenethylamine at orifice 1 voltage of 5 V; (b) benzophenone at orifice 1 voltage of 30 V; (c) diethyl phthalate at orifice 1 voltage of 5 V; (d) metolachlor at orifice 1 voltage of 5 V; (e) progesterone at orifice 1 voltage of 30 V; and (f) sulfometuron methyl ester at orifice 1 voltage of 5 V. Samples were prepared as a 10 μg/mL solution using methylene dichloride as solvent, then sampled with a melting point capillary and air dried for approximately 1 min before DART ionization
Scheme 1 shows the generation of the [M+H]+ ions that are observed in the N2 DART-MS spectra, without any dopant present. The initial step of N2 DART is Penning ionization [33] in which a metastable nitrogen species (including nitrogen atoms at both ground doublet state and excited quartet state), i.e., N2* or N*, transfers energy to molecule M (Reaction 1), resulting in the formation of a molecular ion M+• and an electron e−. This reaction will occur for the majority of organic molecules because their IEs are lower than the internal energy of metastable nitrogen species, i.e., N2* or N*. The M+• ions of most polar compounds then yield [M+H]+ ions through self-protonation (Reaction 2), because their PAs, i.e., PA(M), are stronger than that of their deprotonated radical, e.g., PA([M-H]•) [17].
Generation of [M + NH4]+ Ions
The [M+18]+ ions were previously observed by N2 DART-MS and assigned as water adducts [21]. In our experiments, they were identified as [M+NH4]+ ions through accurate mass measurements within ±2 mDa errors. For some compounds, e.g., PEG 200 (Figure 1c) and THF (Supplementary Figure 2Sb), relevant ammonium adducts coexisted with commonly recognizable [M+H]+ and [M+H-H2O]+ ions, which were used as calibrant so that their identity could not be mistaken. Four ions of acetaldehyde at m/z 115, 132, 150, and 194 were previously assigned as [M++3H2O+NH3], [M++3H2O+2NH3], [M++4H2O+NH3], and [2M++4H2O+2NH3], respectively [21]. In our experiments, acetaldehyde was especially selected and analyzed by N2 DART-MS at orifice 1 voltages of 5, 10, 15, 20, and 30 V to look for relevant ions. Figure 4 shows a N2 DART-MS spectrum of acetaldehyde after calibration with the use of the calibrant, i.e., PEG 200 10% (v/v) solution in methylene chloride, at 15 V orifice 1 voltage. The four ions at m/z 115, 132, 150, and 194 were identified as [3M+H-H2O]+, [3M+NH4-H2O]+, [3M+NH4]+, and [4M+NH4]+ with mass errors of 0.5, 0.2, 0.3, and 0.1 mDa, respectively, after accurate mass measurement (Supplementary Table S6).
In Figure 2a, the polar solvents along the x-axis, i.e., methanol, ethanol, acetonitrile, 2-propanol, acetone, THF, ethyl acetate, DMSO, and DMF, are ordered by increasing PAs, i.e., 754, 776, 779, 793, 812, 822, 836, 884, and 888 kJ/mol, respectively [32]. Therefore, Figure 2a indicates that as the PA of the polar solvents increased and approached the PA of ammonia, i.e., 854 kJ/mol, more and more [M+NH4]+ ions were generated. These polar solvents included methanol, ethanol, acetonitrile, 2-propanol, acetone, THF, and ethyl acetate. They were unable to deprotonate NH4+ due to their weaker PA values than ammonia, but their functional groups were able to form hydrogen bonds with ammonia. On the other hand, DMSO and DMF were able to deprotonate NH4+ due to their stronger PA values than ammonia; therefore [2M+H]+ and/or [M+H]+ ions, instead of [M+NH4]+ ions, were observed. In addition, it should be noted that abundant [M+NH4]+ ions of methanol, ethanol, acetonitrile, 2-propanol, acetone, THF, and ethyl acetate were not observed under identical conditions with He DART-MS (Supplementary Figure S1c).
Analytes for which ammonium adducts are observed are compounds that do not contain strongly basic amine groups, but usually contain O atoms in the form of carbonyl, ester, ether, and/or hydroxyl functional groups [34]. In the analysis of 1% (v/v) solution of propylamine, isopropylamine, and tert-butylamine in methylene chloride by N2 DART-MS, no [M+NH4]+ ions were observed at 5 V orifice 1 voltage (data not shown). In the analysis of 10 μg/mL solution of D-(+)-glucose in methanol by N2 DART-MS, not only [M+NH4]+ but also ammonium adducts of dehydrated fragments were observed (Figure 5a). In comparison, in the analysis of 1 μg/mL solution of D-(+)-glucose in methanol by He DART-MS, no relevant ions of D-(+)-glucose can be found, though proton adducts of small dehydrated fragments were observed in the analysis of a saturated solution of D-(+)-glucose in methanol by He DART-MS without deliberately introducing ammonia into the DART ion source [17]. In the analysis of 10 μg/mL solution of glyceryl trioctanoate in methylene chloride by N2 DART-MS, highly abundant [M+NH4]+ ion relative to the fragment [M+H-CH3(CH2)6COOH ]+ ion was observed (Figure 5b). In comparison, in the analysis of 1 μg/mL solution of glyceryl trioctanoate in methylene chloride by He DART-MS, highly abundant [M+H-CH3(CH2)6COOH ]+ ion relative to the [M+NH4]+ ion was observed (Supplementary Figure S4). It is noted that all the ions in Figures 4 and 5 were identified within ±2 mDa mass error, except for the [M+NH4]+ ion of glyceryl trioctanoate, which was identified with 9.8 mDa mass error (Supplementary Table S6) because the m/z value of the largest detectable ion from the calibrant, i.e., [HO(C2H4O)9H+NH4]+, was 432.2809, approximately 56 Da lower. Finally, it should be indicated that while the abundance of the [M+NH4]+ ions of He DART-MS could be enhanced by opening a bottle of dilute ammonium hydroxide or holding a cotton swab wetted with dilute ammonium hydroxide aqueous solution near the DART ion source, the same was not observed for N2 DART-MS.
N2 DART-MS spectra of: (a) D-(+)-glucose; and (b) glyceryl trioctanoate at orifice 1 voltage of 5 V. Samples were prepared as a 10 μg/mL solution using methanol and methylene chloride as solvent, respectively, then sampled with a melting point capillary and air dried for approximately 2 and 1 min, respectively, before DART ionization
Scheme 2 shows the generation of [M+NH4]+ ions by N2 DART-MS. First, a metastable nitrogen species transfers energy to NH3 (Reaction 3), resulting in the formation of NH3+• and an electron e−. Then, ionized ammonia yields ammonium through reaction with H2O because H2O has a PA value stronger than that of HO• (Reaction 4). Finally, ammonium is attached to hydrogen-bonding polar compounds with PA lower than NH3 through hydrogen bond.
Ionization of Nonpolar Compounds
The ionization ability of N2 DART towards nonpolar compounds was first tested by analyzing alkanes, including cyclohexane, hexanes, heptane, isooctane, and n-hexadecane at orifice 1 voltages of 5, 10, 30, 50, 80, 100, 120, and 150 V. It was found that the N2 DART-MS spectra of alkanes were characterized with oxygen adducts at 30 V orifice 1 voltage, a normal condition for He DART. The oxygen adducts of alkanes would hydrogen-bond with ammonia at low orifice 1 voltage such as 5 V, but fragment to generate [M–(2m+1)H]+ (m = 0,1…) and other hydrocarbon ions at moderately high orifice 1 voltage such as 80 V. Specifically in the N2 DART-MS spectra of n-hexadecane, [M-H-2H+O]+ and [M-H+2O]+ ions were predominant at orifice 1 voltage of 30 V (Supplementary Figure S5a), while [M–H]+ and other hydrocarbon ions such as C7H11+, C5H9+, C4H7+, and C3H5+ were predominant at orifice 1 voltage of 80 V (Supplementary Figure S5b). In contrast, the He DART-MS spectra of n-hexadecane was predominant by [M–H]+ and other hydrocarbon ions at orifice 1 voltage of 20 V [14]. It is noted that hydride abstraction ions were also observed in N2 DART-MS spectra of polar compounds with hydrocarbon chains, e.g. THF (Supplementary Figure S2b).
Scheme 3 shows the generation of [M–(2m+1)H]+ (m = 0,1…) ions and oxygen adducts by N2 DART-MS, which is typical for alkanes. First, a metastable nitrogen species transfers energy to NO (Reaction 6), resulting in the formation of NO+. The generation of NO+ is significant due to its high reactivity, causing a variety of ion-molecule reactions, including charge exchange, hydrogen abstraction, and hydride abstraction (Reaction 7) [17, 35]. It is suspected that NO+ may arise by ionization of neutral NO, produced in a neutral atom reaction involving O atoms produced by metastable nitrogen species [17]. Similar ion-neutral atom reaction can further result in the formation of oxygen adducts (Reaction 8).
The ionization ability of N2 DART towards nonpolar compounds was further tested by analyzing benzene and substituted benzenes, including toluene, anisole, and chlorobenzene at orifice 1 voltages of 5, 10, 30, 50, 80, 100, 120, and 150 V. It was found that the N2 DART-MS spectra of benzene and substituted benzenes was dominated by oxygen adducts of their monomers, dimers, and hydrocarbon fragments at 30 V orifice 1 voltage (Supplementary Figure S6a for toluene). These oxygen adducts were able to fragment and generate [M+H]+, M+, [M-H]+, and other hydrocarbon ions at high orifice 1 voltage such as 100 V (Supplementary Figure S6b for toluene). The ionization mechanism appeared to be a mix of Schemes 1 and 3. In contrast, the He DART-MS spectrum of toluene was predominated by M+ and [M-H]+ ions at orifice 1 voltage of 30 V [16].
Finally, the ionization ability of N2 DART towards nonpolar compounds was tested by analyzing polycyclic aromatic hydrocarbons (PAHs) at orifice 1 voltages of 5, 10, 30, 50, 80, 100, 120, and 150 V. It was found that the N2 DART-MS spectra of small PAHs such as naphthalene, fluorene, anthracene, and 9-methylanthracene were similar to benzene and substituted benzenes. However, the N2 DART-MS spectra of large PAHs such as pyrene, benzo(a)pyrene, and dibenzo(ah)anthracene were dominated by [M+H]+ ions and their oxygen adducts. Figure 6 shows N2 DART-MS spectra of 10 μg/mL pyrene in methylene chloride at orifice 1 voltage of 30 V where [M+H]+, [M+H+O]+, and [M+H+2O]+ ions were predominating. In contrast, the He DART-MS spectrum of 1 μg/mL pyrene in methylene chloride was dominated by only [M+H]+ ions at orifice 1 voltage of 30 V, without any oxygen adducts (Supplementary Figure S7). It is noted that oxygen adducts was also observed in N2 DART-MS spectra of polar compounds with aromatic rings, as shown in Figure 3.
Scheme 4 shows the generation of [M+H]+ and [M+H+nO]+ (n = 1,2,…) ions by N2 DART-MS, which is typical for large PAHs. The ionization requires the presence of ammonium, and its generation is described in Scheme 2 (Reactions 3 and 4). Large PAHs are able to deprotonate ammonium because they possess stronger PAs than ammonia (Reaction 9). Then, ion-neutral atom reaction can further result in the formation of oxygen adducts (Reaction 10).
Conclusions
N2 DART-MS appears to be able to efficiently ionize all polar organic compounds, except for a few extremely small ones such as methanol and acetonitrile, to generate either [M+H]+ ions through Penning ionization followed by self-protonation or [M+NH4]+ ions through hydrogen bonding, in which it may find good applications. N2 DART-MS generally caused less in-source fragmentation and could achieve lower LOD in generating [M+NH4]+ ions than He DART-MS. However, our experiments generally suggest that N2 DART-MS had an order of magnitude higher LOD than He DART-MS in generating [M+H]+ ions. The LOD of N2 DART-MS may be improved by using higher nitrogen flow as the maximum set by the DART-100 controller was used in our experiments. Our experiments also showed lower desorption capability of N2 compared with He DART-MS, which may be improved to achieve better LOD. Nevertheless, in consideration of the looming helium shortage over the past 10 years [27], N2 DART-MS would be a good alternative to He DART-MS in the analysis of polar compounds when needed. Especially, N2 DART-MS should be more appropriate for the analysis of low molecular weight polar compounds than He DART-MS because of typically two orders magnitude weaker background mass spectra. N2 DART-MS should be also more appropriate than He DART-MS when helium is not readily available, e.g., during space mission [21] and other onsite analysis with portable mass spectrometers.
References
Cody, R.B., Laramee, J.A., Durst, H.D.: Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 77, 2297–2302 (2005)
Chernetsova, E.S., Morlock, G.E.: Determination of drugs and drug-like compounds in different samples with direct analysis in rreal time mass spectrometry. Mass Spectrom. Rev. 30, 875–883 (2011)
Harris, G.A., Galhena, A.S., Fernandez, F.M.: Ambient sampling/ionization mass spectrometry: applications and current trends. Anal. Chem. 83, 4508–4538 (2011)
Zhang, J.L., Huo, F.F., Zhou, Z.G., Bai, Y., Liu, H.W.: The principles and applications of an ambient ionization method-direct analysis in real time (DART). Prog. Chem. 24, 101–109 (2012)
Monge, M.E., Harris, G.A., Dwivedi, P., Fernandez, F.M.: Mass spectrometry: recent advances in direct open air surface sampling/ionization. Chem. Rev. 113, 2269–2308 (2013)
Gross, J.H.: Direct analysis in real time–a critical review on DART-MS. Anal. Bioanal. Chem. 406, 63–80 (2014)
Lesiak, A.D., Shepard, J.R.E.: Recent advances in forensic drug analysis by DART-MS. Bioanalysis. 6, 819–842 (2014)
Ferreira, C.R., Yanne, K.E., Jarmusch, A.K., Pirro, V., Ouyang, Z., Cooks, R.G.: Ambient ionization mass spectrometry for point-of-care diagnostics and other clinical measurements. Clin. Chem. 62, 99–110 (2016)
Smoluch, M., Mielczarek, P., Silberring, J.: Plasma-based ambient ionization mass spectrometry in bioanalytical sciences. Mass Spectrom. Rev. 35, 22–34 (2016)
Cody, R.B., Dane, A.J.: Direct analysis in real time (DART). In: Domin, M., Cody, R. (Eds.) Ambient ionization mass spectrometry. Royal Soc. Chem. (2014)
Musselman, B., Tice, J., Crawford, E.: Enabling automated sample analysis by direct analysis in real time (DART) mass spectrometry. In: Domin, M., Cody, R. (Eds.) Ambient ionization mass spectrometry. Royal Soc. Chem. (2014)
Perez, J.J., Harris, G.A., Chipuk, J.E., Brodbelt, J.S., Green, M.D., Hampton, C.Y.: Transmission-mode direct analysis in real time and desorption electrospray ionization mass spectrometry of insecticide-treated bednets for malaria control. Analyst. 135, 712–719 (2010)
Edison, S.E., Lin, L.A., Gamble, B.M., Wong, J., Zhang, K.: Surface swabbing technique for the rapid screening for pesticides using ambient pressure desorption ionization with high-resolution mass spectrometry. Rapid Commun. Mass Spectrom. 25, 127–139 (2011)
Cody, R.B.: Observation of molecular ions and analysis of nonpolar compounds with the direct analysis in real time ion source. Anal. Chem. 81, 1101–1107 (2009)
Song, L.G., Dykstra, A.B., Yao, H.F., Bartmess, J.E.: Ionization mechanism of negative ion-direct analysis in real time: a comparative study with negative ion-atmospheric pressure photoionization. J. Am. Soc. Mass Spectrom. 20, 42–50 (2009)
Song, L.G., Gibson, S.C., Bhandari, D., Cook, K.D., Bartmess, J.E.: Ionization mechanism of positive-ion direct analysis in real time: a transient microenvironment concept. Anal. Chem. 81, 10080–10088 (2009)
Song, L.G., Bartmess, J.E.: Ionization mechanism of direct analysis in real time (DART). In: Domin, M., Cody, R. (Eds.) Ambient ionization mass spectrometry. Royal Soc. Chem. (2014)
Baldwin, K.G.H.: Metastable helium: atom optics with nano-grenades. Contemp. Phys. 46, 105–120 (2005)
Borges, D.L.G., Sturgeon, R.E., Welz, B., Curtius, A.J., Mester, Z.: Ambient mass spectrometric detection of organometallic compounds using direct analysis in real time. Anal. Chem. 81, 9834–9839 (2009)
Keelor, J.D., Dwivedi, P., Fernandez, F.M.: An effective approach for coupling direct analysis in real time with atmospheric pressure drift tube ion mobility spectrometry. J. Am. Soc. Mass Spectrom. 25, 1538–1548 (2014)
Dwivedi, P., Gazda, D.B., Keelor, J.D., Limero, T.F., Wallace, W.T., Macatangay, A.V., et al.: Electro-thermal vaporization direct analysis in real time-mass spectrometry for water contaminant analysis during space missions. Anal. Chem. 85, 9898–9906 (2013)
Harris, G.A., Kwasnik, M., Fernandez, F.M.: Direct analysis in real time coupled to multiplexed drift tube ion mobility spectrometry for detecting toxic chemicals. Anal. Chem. 83, 1908–1915 (2011)
Nah, T., Chan, M., Leone, S.R., Wilson, K.R.: Real Time in Situ Chemical Characterization of submicrometer organic particles using direct analysis in real time-mass spectrometry. Anal. Chem. 85, 2087–2095 (2013)
Jorabchi, K., Hanold, K., Syage, J.: Ambient analysis by thermal desorption atmospheric pressure photoionization. Anal. Bioanal. Chem. 405, 7011–7018 (2013)
Shi, X.Y., Su, R., Yang, H.M., Lian, W.H., Wan, X.L., Liu, S.Y.: Detection of pharmaceuticals by nitrogen direct analysis in real time mass spectrometry. Chem. J. Chin. Univ.-Chin. 38, 362–368 (2017)
Dumlao, M., Khairallah, G.N., Donald, W.A.: Internal energy deposition in dielectric barrier discharge ionization is significantly lower than in direct analysis in real-time mass spectrometry. Aust. J. Chem. 70, 1219–1226 (2017)
Halperin, W.P.: The impact of helium shortages on basic research. Nat. Phys. 10, 467–470 (2014)
Gilmore, F.R.: Potential energy curves for N2, NO, O2, and corresponding ions. J. Quant. Spectrosc. Rad. Trans. 5, 369–390 (1965)
Slanger, T.G.: Reactions of electronically excited diatomic molecules. In: Fontijn, A., Clyne, M.A.A. (eds.) Reactions of small transient species, kinetics, and energetics. Academic Press, New York (1983)
Cernogora, G., Hochard, L., Touzeau, M., Matos Ferreira, C.: Population of N2 (A 3Σ+ u) metastable states in a pure nitrogen glow discharge. J. Phys. B At. Mol. Phys. 14, 2977–2987 (1981)
Foner, S.N., Hudson, R.L.: Mass spectrometric studies of metastable nitrogen atoms and molecules in active nitrogen. J. Chem. Phys. 37, 1662 (1962)
NIST Standard Reference Database Number 69. http://webbook.nist.gov/chemistry/ (accessed August 11, 2017).
Penning, F.M.: Ionization by metastable atoms. Naturwissenschaften. 15, 818–818 (1927)
Bartmess, J.E.: Gas-phase equilibrium affinity scales and chemical ionization mass-spectrometry. Mass Spectrom. Rev. 8, 297–343 (1989)
Harrison, A.G.: Chemical ionization mass spectrometry. CRC Press, Boca Raton, FL (1983)
Acknowledgments
L.S. greatly appreciates the financial support of this research by the Lucas Research Grant Program from Forensic Sciences Foundation (FSF) and the University Research Council (URC) Grant from Western Illinois University (WIU).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 485 kb)
Rights and permissions
About this article
Cite this article
Song, L., Chuah, W.C., Lu, X. et al. Ionization Mechanism of Positive-Ion Nitrogen Direct Analysis in Real Time. J. Am. Soc. Mass Spectrom. 29, 640–650 (2018). https://doi.org/10.1007/s13361-017-1885-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-017-1885-7