Abstract
Reported herein is a simple method of performing single analyzer precursor ion scans in a linear quadrupole ion trap using orthogonal double resonance excitation. A first supplementary AC signal applied to the y electrodes is scanned through ion secular frequencies in order to mass-selectively excite precursor ions while, simultaneously, a second fixed-frequency AC signal is applied orthogonally on the x electrodes in order to eject product ions of selected mass-to-charge ratios towards the detector. The two AC signals are applied orthogonally so as to preclude the possibility of (1) inadvertently ejecting precursor ions into the detector, which results in artifact peaks, and (2) prevent beat frequencies on the x electrodes from ejecting ions off-resonance. Precursor ion scans are implemented while using the inverse Mathieu q scan for easier mass calibration. The orthogonal double resonance experiment results in single ion trap precursor scans with far less intense artifact peaks than when both AC signals are applied to the same electrodes, paving the way for implementation of neutral loss scanning in single ion trap mass spectrometers.

ᅟ
Similar content being viewed by others
Introduction
Miniature mass spectrometers [1, 2] are desirable analytical tools for many specialized applications ranging from pharmaceutical reaction monitoring [3] to forensics [4,5,6], chemical agent analysis [7,8,9,10,11], and planetary exploration [12, 13]. Because the quadrupole ion trap is tolerant of mTorr pressures and electric field imperfections and also has single analyzer product ion MS/MS capabilities [14, 15], it is the ideal mass analyzer for miniaturization. Hence, miniature instruments have increasingly been trending toward quadrupole ion traps over higher-resolution but more complex alternatives [2, 16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31].
Even so, quadrupole ion traps by themselves do not currently perform precursor ion or neutral loss scans, two of the three main types of MS/MS experiments [32]. This is an unfortunate omission since both types of scans are particularly useful for analyzing complex mixtures for ions with similar functional groups [33,34,35,36,37,38,39,40,41,42,43,44]. Planetary exploration and forensics, in particular, may benefit from single analyzer precursor ion scanning capabilities since small organic molecules such as aryls with common substituents and various classes of drugs often have fragment ions in common. For planetary exploration, a data-independent precursor ion scan would help increase the efficiency of ion utilization and decrease power and sample consumption compared with obtaining product ion spectra of multiple precursor ions (and thereby reconstructing the precursor ion spectrum). Data-dependent precursor ion scans would require multiple ion injections from the same source material.
The difficulty in performing a precursor scan in a single analyzer stems from its three requirements: (1) mass selection of precursor ions, (2) fragmentation of the mass-selected precursors, and (3) mass analysis of the resulting product ions. Conventionally, the simultaneous mass selection of precursor and product ions has required multi-analyzer instruments such as the triple quadrupole (or pentaquadrupole) [45, 46]. Other instruments that can perform precursor ion scans are multisector mass spectrometers, [33] quadrupole/time-of-flight instruments [36, 38, 40], and triple quadrupoles used in tandem with ion traps (e.g., Q-traps) [42]. As mentioned previously, none of these configurations is ideal for miniaturization due to the sheer size, power consumption, and complexity of multi-analyzer mass spectrometers.
It is commonly accepted that single quadrupole ion traps can perform product ion scans but that they do not have data-independent precursor or neutral loss scanning capabilities. However, some two and a half decades ago, Yost et al. demonstrated, in principle, precursor ion scanning in a 3D quadrupole ion trap using a double resonance technique [47]. A first scanned AC frequency was applied to the endcaps in order to mass selectively excite precursor ions, thereby providing the functionality of the first and second quadrupole in a triple quadrupole system. The function of the third quadrupole, detection of a particular product ion m/z, was implemented in the ion trap with a second AC frequency fixed on the product ion of interest. Ideally, a signal would only occur at the detector when a product ion with m/z value corresponding to this second frequency was produced by fragmentation of a precursor ion. Because the precursor ions are fragmented mass selectively in time with a swept frequency sinusoid, the correlation between precursor and product ion – as well as mass information – is preserved in the ejection time of the product ion (in principle, a neutral loss scan can similarly be performed by scanning both AC frequencies at a constant mass offset, but this has not been demonstrated).
Since this initial development, precursor and neutral loss spectra in single ion traps have almost always been recorded either using data-dependent acquisition [48, 49], requiring multiple ion injections and thus multiple mass scans, or using data-independent activation methods that also require multiple ion injections [50], or by methods which rely on data deconvolution [51]. Furthermore, none of these methods are single scans; rather, they all require multiple scans. For miniature and portable instruments – especially for planetary exploration and other situations where power and sample mass are limited – these methods are unsuitable.
We have previously implemented a double resonance precursor ion scan based on AC frequency scans using the Mini 12 [52] miniature linear ion trap mass spectrometer developed at Purdue University [53]. In the process of optimizing this scan mode, many artifact peaks were observed and, furthermore, mass calibration was difficult because the AC frequency was scanned linearly, giving a nonlinear relationship between m/z and time [54]. We postulated that artifact peaks were caused by (1) beat frequencies resulting from the application of multiple AC frequencies on the same pair of rods, and (2) accidental ejection of precursor ions as the first AC frequency was scanned. In this follow-up publication, we demonstrate single analyzer precursor scans in a linear quadrupole ion trap using orthogonal double resonance excitation. In comparison to the previously demonstrated parallel excitation experiment, orthogonal excitation largely removes artifact peaks. Precursor ion scanning is also demonstrated in conjunction with the inverse Mathieu q scan, which forces an approximately linear relationship between m/z and time, thereby simplifying mass calibration compared with the linear frequency sweep case.
Experimental
Chemicals
Amphetamine, methamphetamine, 3,4-methylenedioxyamphetamine, and 3,4-methylenedioxymethamphetamine were purchased from Cerilliant (Round Rock, TX, USA) and dissolved in methanol. Final drug concentrations of 1–5 ppm were used for all experiments. Pierce ESI calibration mixture containing caffeine, the peptide MRFA, and the phosphazine Ultramark 1621 was purchased from Thermo Fisher (Rockford, IL, USA). Cesium hydrogencarbonate, tridecafluoroheptanoic acid (TFHA), monobutyl phthalate (MBP), and sodium sulfate were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA) and were dissolved in 50:50 methanol:water at a concentration of 3 mM. For generating CsTFHA or CsMBP cluster ions, the cesium hydrogencarbonate and either TFHA or MBP were dissolved in the same solution and electrosprayed as is.
Ionization
Ions were generated by nanoelectrospray ionization (nESI) at 1500 V using 5 μm nanospray tips pulled from borosilicate glass capillaries (1.5 mm o.d., 0.86 i.d.; Sutter Instrument Co., Novato, CA, USA) by a Flaming/Brown micropipette puller (model P-97; Sutter Instrument Co.). The flow rate in the nanoelectrospray performed here is on the order of 10 nL/min [55]. The positive ion mode was used for all experiments. Ion injection time was generally set at 50 ms but was manually optimized to prevent trap overloading. Automatic gain control was not used in this study.
Instrumentation
A Thermo Instruments (San Jose, CA, USA) LTQ linear ion trap mass spectrometer running LTQ XL 2.7 software was used for all experiments. The commercial rf coil was modified so that dipolar AC signals could be applied simultaneously to both the x and y rods while also applying the high voltage trapping rf signal in a quadrupolar manner. Otherwise the instrument was used as supplied commercially.
Generally, AC frequency scanning is performed at constant trapping (rf frequency and amplitude) conditions. However, ion trap control language (ITCL) code was not available, so instead, AC frequency scanning was performed during an Ultrazoom scan. The Ultrazoom scan is the closest available approximation to constant rf conditions, as the rf amplitude is swept slowly at a rate corresponding to 27 Da/s. For experiments performed here, the starting Ultrazoom mass is reported because rf voltage read-backs are not provided by the manufacturer.
Orthogonal precursor ion scans were accomplished by applying on the y electrodes a swept frequency sinusoid for mass-selective precursor ion excitation while simultaneously applying to the x electrodes a second fixed-frequency signal corresponding to the secular frequency and hence m/z of a particular product ion [47, 53]. The result of this orthogonal double resonance [56, 57] experiment is a single analyzer precursor scan. All supplemental AC waveforms were supplied by a Keysight 33612A arbitrary waveform generator (Newark element14, Chicago, IL, USA). Channel 1 supplied the ejection signal to the x electrodes and channel 2 supplied the swept frequency resonance excitation signal to the y electrodes. The generator was triggered at the start of the mass scan using the triggers built in to the LTQ Tune “Diagnostics” menu.
Results and Discussion
Single Analyzer Precursor Ion Scans
In order to perform a single analyzer precursor scan, the functions of the three quadrupoles in a triple quadrupole mass spectrometer must be condensed onto a single analyzer. For the quadrupole ion trap, this conundrum translates into the use of dual supplemental AC frequencies for ion excitation and ejection. A first frequency (“scanned excitation frequency” in Figure 1a) is scanned through a range of ion secular frequencies in order to excite the precursor ions in the trap mass selectively as a function of time. A second frequency (“fixed ejection frequency” in Figure 1a), set on the frequency corresponding to the product ion of interest, is used to eject product ions of a particular m/z. The resulting scan table is shown in Figure 1b. Because ion secular frequencies depend on the trapping rf voltage and frequency, both must be kept constant during the precursor scan. Instead of using an rf ramp for mass scanning, the first AC frequency is swept either linearly or nonlinearly, a selection that affects mass calibration. The second frequency, the product ion ejection frequency, is fixed. In this study, the amplitudes of both precursor excitation and product ejection waveforms were kept constant, although they could have been varied in order to alter the normalized collision energy that each precursor ion experiences or to vary with precursor ion potential well depth. However, the AC amplitudes will also affect mass calibration, so they were not varied in this work.
Single analyzer precursor ion scans in a linear quadrupole ion trap using orthogonal double resonance excitation: (a) illustration of precursor ion scanning on the Mathieu stability diagram, where a first AC frequency is scanned through ion secular frequencies to excite precursors while simultaneously a second AC signal is fixed on a particular product ion, (b) scan table for the precursor scan, and (c) schematic of orthogonal AC signals applied to the rods of an LTQ linear ion trap
Orthogonal Versus Parallel Precursor Ion Scans
The key aspect of the current precursor scan experiment that differentiates it from previous implementations is the use of the orthogonal dimension for ion excitation. Few studies have used the y dimension in linear ion traps for mass selective operations because the detector lies in the orthogonal x direction. Rhombic ion excitation is one such case where orthogonal excitation signals are used to resonantly eject ions [58]. The resulting rhombic ion motion was shown to decrease space charge effects compared with typical single-direction resonance ejection. Here we use orthogonality for double resonance excitation.
The orthogonality in the precursor scan is critical to removing artifact peaks, which can result from beat frequencies as well as unintended precursor ion ejection caused by the excitation AC frequency sweep. For example, Figure 2a shows a full scan mass spectrum from 200 to 140 kHz of the peptide MRFA from the Pierce ESI LTQ calibration mixture, which also contains caffeine and Ultramark 1621. The orthogonal precursor scan of m/z 288 (shown in Figure 2b), the most abundant fragment of MRFA in LTQ CID (see Table 1 for precursor and product ion relationships), returns a single peak (m/z 524) corresponding to the time at which the precursor ion forming the m/z 288 product ion fragments. Using the ejection time of MRFA (ejection time 0.169 s in the precursor scan compared with 0.17 s in the full mass scan) as a reference for its secular frequency, 166 kHz, we calculate the secular frequency of m/z 288 as 335 kHz, in close agreement with the experimental value of 331 kHz. Note that because the product ions are generated far from the center of the trap, they may experience substantial secular frequency shifts compared with their precursor ions, which can contribute to differences between calculated and experimentally observed secular frequencies. Also, ions are not ejected on resonance; rather, they are ejected slightly beforehand, which can further increase the error in secular frequency calculations based on ejection time. Furthermore, because the rf amplitude is being ramped, ion secular frequencies will shift slightly during the scan, increasing the error in calculated secular frequencies. This effect, in particular, likely has the greatest effect on the results presented here. Nonetheless, errors within perhaps 10 kHz were chosen to be acceptable in these calculations.
Comparison of parallel and orthogonal precursor ion scans. (a) Shows full AC scan mass spectrum of MRFA, (b) shows orthogonal precursor scan of m/z 288, and (c) shows parallel precursor scan of m/z 288 wherein both excitation and ejection signals were applied to the x electrodes. The excitation signal was scanned from 200 to 140 kHz over 0.3 s with an amplitude of 1.1 Vp-p. The ejection signal was set at a frequency of 331 kHz, 1 Vp-p. The Ultrazoom scan started at m/z 240
The parallel variant of the precursor ion scan, in which both supplemental AC waveforms are applied to the x electrodes, returns the result in Figure 2c. Essentially the parallel experiment records a full scan mass spectrum because the excitation AC waveform ejects the precursor ions into the detector. If the excitation amplitude is decreased from 1.1 Vp-p to 300 mVp-p, only the MRFA peak is left. However, the high signal intensity (102 counts) indicates that the precursor ion is still being ejected, and there is no way to unambiguously determine the relative contributions of the full scan and the precursor ion scan. Hence, although the orthogonal variant appears to lose sensitivity (a factor of 100 in this case), it returns less ambiguous results than the parallel variant. Moreover, the 100× drop in sensitivity should be interpreted as a ‘worst case scenario’ because we are unfortunately forced by commercial operating system constraints to ramp the rf very slowly on our commercial instrument, and as the rf amplitude increases the ions’ secular frequencies increase. The AC frequency is scanning from high to low (in the opposite direction of the shift in ion frequencies via the rf ramp) and hence the time each ion has on resonance is quite short. By keeping the rf constant and by scanning more slowly, we expect that much higher sensitivity will be observed.
Application to Amphetamines
Many amphetamines as well as designer drugs [59,60,61] give similar fragments in their product ion scans [62] and the protonated molecules also fragment quite readily in ion traps. The latter characteristic is particularly important for precursor ion scans in ion traps because of the slow heating CID process [63] and also because the precursor ions are on resonance for a very short period of time, which can lead to limited (if any) fragmentation. The short on-resonance time is further exacerbated by the slow rf ramp necessarily applied using the LTQ, which causes ion secular frequencies to drift. On a more customized instrument, the rf ramp would not be an obstacle.
A full AC scan mass spectrum of a mixture of amphetamine (amp), methamphetamine (map), 3,4-methylenedioxyam-phetamine (mda), and 3,4-methylenedioxymethamphetamine (mdma) is shown in Figure 3a. Because amphetamine and methamphetamine both fragment readily to m/z 119, the precursor ion scan shows both peaks. The calculated secular frequency of m/z 119 under these conditions is 200 kHz, in agreement with the experimental value of 206 kHz. Under these conditions, the product ion’s secular frequency is calculated to shift by 7 kHz (from ~202.5 kHz to ~209.1 kHz) during the rf scan period between ejection of amp and mdma, which can contribute to both loss of signal intensity and the error in secular frequency calculations. A similar precursor ion scan of m/z 163, the primary fragment of both mda and mdma, again yields both corresponding peaks. Once again the calculated and experimental secular frequency of the product ion agreed within 10 kHz.
Orthogonal double resonance precursor ion scanning of amphetamines. (a) Full AC frequency scan mass spectrum of amphetamine (amp), methamphetamine (map), 3,4-methylenedioxyamphetamine (mda), and 3,4-methylenedioxymethamphetamine (mdma), (b) orthogonal precursor ion scan of m/z 119, and (c) orthogonal precursor ion scan of m/z 163. The excitation signal on the y electrodes was scanned from 360 to 100 kHz over 400 ms with an amplitude of 260 mVp-p. The ejection signal on the x electrodes was set at (b) 206 kHz, 260 mVp-p, or (c) 151.6 kHz, 240 mVp-p. The Ultrazoom scan started at m/z 60. For (b) and (c), resonance ejection background signal was subtracted from the raw signal
Multigenerational Precursor Ion Scans
One of the unique features of quadrupole ion trap precursor ion scanning is direct access to multigenerational scans without need for another analyzer. We and others have previously documented multigenerational collision-induced dissociation scans wherein multiple generations of fragment ions can be produced by sweeping the rf amplitude from high to low while a fixed excitation frequency is applied for CID [62, 64,65,66], or by applying multiple resonance frequencies corresponding to, for example, fixed neutral loss fragments [50]. A similar experiment can be performed in ion traps by sweeping the AC frequency in the unconventional direction (low to high) so that high mass ions are first to fragment, followed by their product ions [66]. For example, Figure 4a shows a full AC scan mass spectrum from 100 to 360 kHz (the opposite direction to that used in Figure 3). Resolution and sensitivity are much worse in this mode due to ion frequency shifts, as has been documented previously [67,68,69,70,71,72], and here further compounded by the rf ramp.
Multigenerational precursor ion scanning in a single linear ion trap. (a) Shows full scan mass spectrum from high to low mass (low to high frequency), (b) shows single generation precursor ion scan of m/z 119, and (c) shows multigenerational precursor ion scan of m/z 91 (a product ion of m/z 119 and second generation product ion of m/z 136). The excitation signal on the y electrodes was scanned from 100 to 360 kHz with an amplitude of 240 mVp-p over 0.4 s. The ejection signal on the x electrodes was set at (b) 188 kHz (240 mVp-p) or (c) 261 kHz (240 mVp-p). The Ultrazoom scan started at m/z 90
A precursor ion scan of m/z 119 gives only protonated amphetamine (m/z 136) (and a broad peak due to its unwanted rf ramp resonance ejection). Nonetheless, using the relationship m/z 136 ->119 -> 91, we can perform a multigenerational precursor ion scan by setting the product ion ejection frequency on m/z 91 (experimentally 261 kHz, calculated 253 kHz) and observing the transition m/z 119 -> 91 at t = 0.135 s, which corresponds to a secular frequency of 187 kHz for the m/z 119 ion, in agreement with the calculated value. This peak is not observed in the full mass scan because it can only be produced by first fragmenting m/z 136 (at t = 0.095 s) and subsequently fragmenting its product ion m/z 119 (at t = 0.135 s), and finally ejecting the second generation product ion m/z 91. This experiment is akin to setting the third and fifth analyzers in a pentaquadrupole mass spectrometer [46, 73,74,75] on m/z 119 and m/z 91, respectively, and scanning the first quadrupole through a range of precursor ion m/z values. However, the pentaquadrupole is considerably more complex and is not portable or of appropriate size for planetary exploration. Of course, the relationship between m/z 136 and m/z 91 is only clear in the ion trap data when both the single and multigenerational precursor scans are compared (or if the forward and reverse precursor scans are compared). Otherwise, the relationship would be ambiguous.
Precursor Ion Scanning of Cluster Ions
The tightly controlled nature of the commercial LTQ – that is, the forced use of the Ultrazoom scan to approximate a fixed rf amplitude – restricted our experiments to ions that fragment quite readily. Furthermore, there was a lower limit to the AC frequency scan rate, again forced by the use of an rf ramp. With more customized instrumentation, however, the excitation scan could be slowed to give each ion more time on resonance, thereby increasing the quantity of product ions formed. As a result of these restrictions, cluster ions were chosen to demonstrate precursor ion scanning because (1) they fragment readily with very little resonance time, (2) they form similar or identical product ions, and (3) the precursor ions fall over a wide mass range. This last characteristic made simple mass calibration possible, as discussed later.
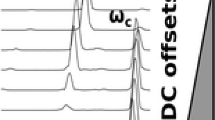
Precursor ion scans were performed in the positive ion mode on cesium tridecafluoroheptanoic acid clusters (Figure 5a–d), cesium monobutyl phthalate clusters (Figure 5e, f), and on sodium sulfate clusters (Figure 6). In the case of the cesium-containing clusters, at both high and low m/z clean precursor ion spectra were obtained (b, d, and f). These spectra mimicked the full scan mass spectra (a, c, and e) because the clusters in the full scan fragment easily to common product ions. For example, for higher mass CsTFHA clusters, m/z 629 was a common fragment, and for lower mass CsTFHA clusters, m/z 133 was a common fragment. The CsMBP clusters shared m/z 841 as the common product ion; hence, a precursor ion scan of this product ion (Figure 5f) mirrored the full scan data (Figure 5e).
Single analyzer precursor ion scans of cesium salt clusters: (a) full AC frequency scan of CsTFHA clusters from m/z 1000 to 2000, (b) precursor ion scan of m/z 629, (c) full AC scan of CsTFHA clusters from m/z 100 to 500, (d) precursor ion scan of m/z 133, (e) full AC scan of CsMBP clusters from m/z 1000 to 2000, and (f) precursor ion scan of m/z 841
Linear mass calibration in single analyzer precursor ion scans: (a) full AC scan of NaSO4 clusters, (b) nonlinear precursor ion scan of m/z 660 (linear frequency sweep), (c) precursor ion scan of m/z 660 using the inverse Mathieu q scan for excitation, and (d) linear mass calibration for the scan in (c)
Linear Mass Calibration
All of the data shown up to this point were obtained using linear frequency sweeping. Linear frequency sweeping gives each ion the same amount of time on resonance because the scan rate in frequency units per unit time is constant. However, the mass spectral data then must be calibrated using the nonlinear Mathieu parameters, which is not ideal [54]. For another example, a full scan of sodium sulfate cluster ions is shown in Figure 6a. The precursor ion scan of m/z 660 (Figure 6b), a common product ion, gives a single stage MS-like spectrum. However, the nonlinear nature of the relationship between ion secular frequency and time makes calibration non-intuitive. Instead, the excitation waveform can be replaced by a nonlinear frequency sweep in the form of an inverse Mathieu q scan, which excites the precursor ions such that there is an approximately linear relationship between the excited ion’s m/z and time [76]. Figure 6c shows the same precursor ion spectrum wherein the excitation scan was an inverse Mathieu q scan from q = 0.908 to q = 0.15 taken over 300 ms. As shown in panel d, there is an approximately linear relationship between m/z and time, making calibration simple. Note, however, that because the excitation frequency is scanned nonlinearly, the precursor ions will have about the same number of secular oscillations but different amounts of time on resonance, which can affect the relative intensities of the peaks observed.
Conclusions
We have demonstrated precursor ion scanning in a single quadrupole ion trap mass analyzer using an orthogonal double resonance technique. In combination with nanoelectrospray, the data-independent precursor ion scan uses minimal sample yet maintains reasonable sensitivity, characteristics ideal for resource-limited situations (e.g., a miniature ion trap mass spectrometer on Mars).
Orthogonal activation suffers from fewer artifact peaks than parallel activation and, therefore, allows for higher energy deposition into the precursor ions without fear of precursor ion detection. Unlike triple quadrupole and other double analyzer instruments, the choice of scan direction in the ion trap can be used to access higher order precursor scanning experiments by successively fragmenting precursor and product ions in the same excitation sweep. Furthermore, a linear relationship between m/z and time can be observed if the precursor ion excitation scan is an inverse Mathieu q scan. Cluster ions are ideal calibrants for these scans because they share product ions, fragment quite readily, and fall over a wide mass range.
Further work is needed to characterize resolution and sensitivity in order to understand the performance of single analyzer precursor scans compared with multi-analyzer scans.
The results demonstrated here pave the way for further utilization of precursor ion scanning as well as implementation of neutral loss scanning and double resonance multiple reaction monitoring scans in single ion trap mass analyzers using orthogonal excitation. These scans will further solidify the quadrupole ion trap’s emerging standing as the most versatile of mass analyzers as well as the one most amenable to miniaturization.
References
Ouyang, Z., Cooks, R.G.: Miniature mass spectrometers. Annu. Rev. Anal. Chem. 2, 187–214 (2009)
Snyder, D.T., Pulliam, C.J., Ouyang, Z., Cooks, R.G.: Miniature and fieldable mass spectrometers: recent advances. Anal. Chem. 88, 2–29 (2016)
Browne, D.L., Wright, S., Deadman, B.J., Dunnage, S., Baxendale, I.R., Turner, R.M., Ley, S.V.: Continuous flow reaction monitoring using an on-line miniature mass spectrometer. Rapid Commun. Mass Spectrom. 26, 1999–2010 (2012)
O'Leary, A.E., Oberacher, H., Hall, S.E., Mulligan, C.C.: Combining a portable, tandem mass spectrometer with automated library searching – an important step towards streamlined, on-site identification of forensic evidence. Anal. Methods 7, 3331–3339 (2015)
Chen, C.H., Lin, Z., Tian, R., Shi, R., Cooks, R.G., Ouyang, Z.: Real-time sample analysis using a sampling probe and miniature mass spectrometer. Anal Chem. 87, 8867–8873 (2015)
Lawton, Z.E., Traub, A., Fatigante, W.L., Mancias, J., O'Leary, A.E., Hall, S.E., Wieland, J.R., Oberacher, H., Gizzi, M.C., Mulligan, C.C.: Analytical validation of a portable mass spectrometer featuring interchangeable, ambient ionization sources for high throughput forensic evidence screening. J. Am. Soc. Mass Spectrom. (2016). doi:10.1007/s13361-016-1562-2
Smith, J.N., Noll, R.J., Cooks, R.G.: Facility monitoring of chemical warfare agent simulants in air using an automated, field-deployable, miniature mass spectrometer. Rapid Commun. Mass Spectrom. 25, 1437–1444 (2011)
Dumlao, M., Sinues, P.M.-L., Nudnova, M., Zenobi, R.: Real-time detection of chemical warfare agent simulants in forensic samples using active capillary plasma ionization with benchtop and field-deployable mass spectrometers. Anal. Methods 6, 3604 (2014)
Hendricks, P.I., Dalgleish, J.K., Shelley, J.T., Kirleis, M.A., McNicholas, M.T., Li, L., Chen, T.C., Chen, C.H., Duncan, J.S., Boudreau, F., Noll, R.J., Denton, J.P., Roach, T.A., Ouyang, Z., Cooks, R.G.: Autonomous in situ analysis and real-time chemical detection using a backpack miniature mass spectrometer: concept, instrumentation development, and performance. Anal. Chem. 86, 2900–2908 (2014)
Urabe, T., Takahashi, K., Kitagawa, M., Sato, T., Kondo, T., Enomoto, S., Kidera, M., Seto, Y.: Development of portable mass spectrometer with electron cyclotron resonance ion source for detection of chemical warfare agents in air. Spectrochim Acta A Mol. Biomol. Spectrosc. 120, 437–444 (2014)
Giannoukos, S., Brkic, B., Taylor, S., France, N.: Membrane inlet mass spectrometry for homeland security and forensic applications. J. Am. Soc. Mass Spectrom. 26, 231–239 (2015)
Brinckerhoff, W., Danell, R., Van Ameron, F., Pinnick, V., Li, X., Arevalo, R., Glavin, D., Getty, S., Mahaffy, P., Chu, P.: Development of a linear ion trap mass spectrometer (LITMS) investigation for future planetary surface missions. Conference paper. International Workshop on Instrumentation for Planetary Missions, 4–7 Nov. 2014, Greenbelt, MD, United States (2014)
Brinckerhoff, W.B., Pinnick, V.T., van Amerom, F.H.W., Danell, R.M., Arevalo, R.D., Atanassova, M.S., Li, X., Mahaffy, P.R., Cotter, R.J., Goesmann, F., Steininger, H., Team, M.: Mars organic molecule analyzer (MOMA) mass spectrometer for ExoMars 2018 and beyond. Aerospace Conference, 2013 IEEE. Big Sky, MT, USA. 2–9 March 2013 (2013)
Wu, Q., Tian, Y., Li, A., Austin, D.E.: Simulations of electrode misalignment effects in two-plate linear ion traps. Int. J. Mass Spectrom. 393, 52–57 (2015)
Tian, Y., Higgs, J., Li, A., Barney, B., Austin, D.E.: How far can ion trap miniaturization go? Parameter scaling and space-charge limits for very small cylindrical ion traps. J. Mass Spectrom. 49, 233–240 (2014)
Fulford, J.E., March, R.E., Mather, R.E., Todd, J.F.J., Waldren, R.M.: The cylindrical ion trap – a theoretical and experimental study. Can. J. Spectrosc. 25, 85–97 (1980)
Badman, E.R., Johnson, R.C., Plass, W.R., Cooks, R.G.: A miniature cylindrical quadrupole ion trap: simulation and experiment. Anal. Chem. 70, 4896–4901 (1998)
Wells, J.M., Badman, E.R., Cooks, R.G.: A quadrupole ion trap with cylindrical geometry operated in the mass-selective instability mode. Anal. Chem. 70, 438–444 (1998)
Badman, E.R., Cooks, R.G.: A parallel miniature cylindrical ion trap array. Anal. Chem. 72, 3291–3297 (2000)
Ouyang, Z., Wu, G., Song, Y., Li, H., Plass, W.R., Cooks, R.G.: Rectilinear ion trap: concepts, calculations, and analytical performance of a new mass analyzer. Anal. Chem. 76, 4595–4605 (2004)
Gao, L., Song, Q., Patterson, G.E., Cooks, R.G., Ouyang, Z.: Hand-held rectilinear ion trap mass spectrometer. Anal. Chem. 78, 5994–6002 (2006)
Song, Q., Kothari, S., Senko, M.A., Schwartz, J.C., Amy, J.W., Stafford, G.C., Cooks, R.G., Ouyang, Z.: Rectilinear ion trap mass spectrometer with atmospheric pressure interface and electrospray ionization source. Anal. Chem. 78, 718–725 (2006)
Zhang, C., Chen, H., Guymon, A.J., Wu, G., Cooks, R.G., Ouyang, Z.: Instrumentation and methods for ion and reaction monitoring using a non-scanning rectilinear ion trap. Int. J. Mass Spectrom. 255/256, 1–10 (2006)
Fico, M., Yu, M., Ouyang, Z., Cooks, R.G., Chappell, W.J.: Miniaturization and geometry optimization of a polymer-based rectilinear ion trap. Anal. Chem. 79, 8076–8082 (2007)
Lammert, S.A., Plass, W.R., Thompson, C.V., Wise, M.B.: Design, optimization and initial performance of a toroidal rf ion trap mass spectrometer. Int. J. Mass Spectrom. 212, 25–40 (2001)
Lammert, S.A., Rockwood, A.A., Wang, M., Lee, M.L., Lee, E.D., Tolley, S.E., Oliphant, J.R., Jones, J.L., Waite, R.W.: Miniature toroidal radio frequency ion trap mass analyzer. J. Am. Soc. Mass Spectrom. 17, 916–922 (2006)
Austin, D.E., Wang, M., Tolley, S.E., Maas, J.D., Hawkins, A.R., Rockwood, A.L., Tolley, H.D., Lee, E.D., Lee, M.L.: Halo ion trap mass spectrometer. Anal. Chem. 79, 2927–2932 (2007)
Contreras, J.A., Murray, J.A., Tolley, S.E., Oliphant, J.L., Tolley, H.D., Lammert, S.A., Lee, E.D., Later, D.W., Lee, M.L.: Hand-portable gas chromatograph-toroidal ion trap mass spectrometer (GC-TMS) for detection of hazardous compounds. J. Am. Soc. Mass Spectrom. 19, 1425–1434 (2008)
Peng, Y., Hansen, B.J., Quist, H., Zhang, Z., Wang, M., Hawkins, A.R., Austin, D.E.: Coaxial ion trap mass spectrometer: concentric toroidal and quadrupolar trapping regions. Anal. Chem. 83, 5578–5584 (2011)
Wu, Q., Li, A., Tian, Y., Zare, R.N., Austin, D.E.: Miniaturized linear wire ion trap mass analyzer. Anal. Chem. 88(15), 7800–7806 (2016)
Wu, Q., Tian, Y., Li, A., Andrews, D., Hawkins, A.R., Austin, D.E.: A miniaturized linear wire ion trap with electron ionization and single photon ionization sources. J. Am. Soc. Mass Spectrom. 28(5), 859–865 (2017)
Schwartz, J.C., Wade, A.P., Enke, C.G., Cooks, R.G.: Systematic delineation of scan modes in multidimensional mass spectrometry. Anal. Chem. 62, 1809–1818 (1990)
Scrivens, J.H., Rollins, K., Jennings, R.C.K.: The implementation and application of precursor-ion scanning using a four-sector instrument. Rapid Commun. Mass Spectrom. 6, 272–277 (1992)
Gu, M., Turecek, F.: The precursor scan – a new-type of experiment in neutralization-reionization mass-spectrometry. Org. Mass Spectrom. 28, 1135–1143 (1993)
Carr, S.A., Huddleston, M.J., Annan, R.S.: Selective detection and sequencing of phosphopeptides at the femtomole level by mass spectrometry. Anal. Biochem. 239, 180–192 (1996)
Borchers, C., Parker, C.E., Deterding, L.J., Tomer, K.B.: Preliminary comparison of precursor scans and liquid chromatography-tandem mass spectrometry on a hybrid quadrupole time-of-flight mass spectrometer. J. Chromatogr. A 854, 119–130 (1999)
Steen, H., Küster, B., Fernandez, M., Pandey, A., Mann, M.: Detection of tyrosine phosphorylated peptides by precursor ion scanning quadrupole TOF mass spectrometry in positive ion mode. Anal. Chem. 73, 1440–1448 (2001)
Steen, H., Kuster, B., Mann, M.: Quadrupole time-of-flight versus triple-quadrupole mass spectrometry for the determination of phosphopeptides by precursor ion scanning. J. Mass Spectrom. 36, 782–790 (2001)
Bateman, R.H., Carruthers, R., Hoyes, J.B., Jones, C., Langridge, J.I., Millar, A., Vissers, J.P.: A novel precursor ion discovery method on a hybrid quadrupole orthogonal acceleration time-of-flight (Q-TOF) mass spectrometer for studying protein phosphorylation. J. Am. Soc. Mass Spectrom. 13, 792–803 (2002)
Ritchie, M.A., Gill, A.C., Deery, M.J., Lilley, K.: Precursor ion scanning for detection and structural characterization of heterogeneous glycopeptide mixtures. J. Am. Soc. Mass Spectrom. 13, 1065–1077 (2002)
Rappsilber, J., Friesen, W.J., Paushkin, S., Dreyfuss, G., Mann, M.: Detection of arginine dimethylated peptides by parallel precursor ion scanning mass spectrometry in positive ion mode. Anal. Chem. 75, 3107–3114 (2003)
Sandra, K., Devreese, B., Van Beeumen, J., Stals, I., Claeyssens, M.: The Q-Trap mass spectrometer, a novel tool in the study of protein glycosylation. J. Am. Soc. Mass Spectrom. 15, 413–423 (2004)
Ejsing, C.S., Duchoslav, E., Sampaio, J., Simons, K., Bonner, R., Thiele, C., Ekroos, K., Shevchenko, A.: Automated identification and quantification of glycerophospholipid molecular species by multiple precursor ion scanning. Anal. Chem. 78, 6202–6214 (2006)
Iglesias, A.H., Santos, L.F., Gozzo, F.C.: Identification of cross-linked peptides by high-resolution precursor ion scan. Anal. Chem. 82, 909–916 (2010)
Yost, R.A., Enke, C.G.: Triple quadrupole mass spectrometry for direct mixture analysis and structure elucidation. Anal. Chem. 51, 1251–1264 (1979)
Eberlin, M.N.: Triple-stage pentaquadrupole (QqQqQ) mass spectrometry and ion/molecule reactions. Mass Spectrom. Rev. 16, 113–144 (1997)
Johnson, J.V., Pedder, R.E., Yost, R.A.: MS-MS parent scans on a quadrupole ion trap mass-spectrometer by simultaneous resonant excitation of multiple ions. Int. J. Mass Spectrom. Ion Processes 106, 197–212 (1991)
McClellan, J.E., Quarmby, S.T., Yost, R.A.: Parent and neutral loss monitoring on a quadrupole ion trap mass spectrometer: screening of acylcarnitines in complex mixtures. Anal. Chem. 74, 5799–5806 (2002)
Swaney, D.L., McAlister, G.C., Coon, J.J.: Decision tree-driven tandem mass spectrometry for shotgun proteomics. Nat. Methods 5, 959–964 (2008)
Schroeder, M.J., Shabanowitz, J., Schwartz, J.C., Hunt, D.F., Coon, J.J.: A neutral loss activation method for improved phosphopeptide sequence analysis by quadrupole ion trap mass spectrometry. Anal. Chem. 76, 3590–3598 (2004)
Bern, M., Finney, G., Hoopmann, M.R., Merrihew, G., Toth, M.J., MacCoss, M.J.: Deconvolution of mixture spectra from ion-trap data-independent-acquisition tandem mass spectrometry. Anal. Chem. 82, 833–841 (2010)
Li, L., Chen, T.C., Ren, Y., Hendricks, P.I., Cooks, R.G., Ouyang, Z.: Mini 12, miniature mass spectrometer for clinical and other applications – introduction and characterization. Anal. Chem. 86, 2909–2916 (2014)
Snyder, D.T., Pulliam, C.J., Cooks, R.G.: Single analyzer precursor scans using an ion trap. Rapid Commun. Mass Spectrom. 30, 800–804 (2016)
Snyder, D.T., Pulliam, C.J., Cooks, R.G.: Calibration procedure for secular frequency scanning in an ion trap. Rapid Commun. Mass Spectrom. 30, 1190–1196 (2016)
Juraschek, R., Dulcks, T., Karas, M.: Nanoelectrospray – more than just a minimized-flow electrospray ionization source. J. Am. Soc. Mass Spectrom. 10, 300–308 (1999)
Fulford, J.E., Nhu-Hoa, D., Hughes, R.J., March, R.E., Bonner, R.F., Wong, G.J.: Radio-frequency mass selective excitation and resonant ejection of ions in a three-dimensional quadrupole ion trap. J. Vac. Sci. Technol. 17, 829–835 (1980)
Asam, M.R., Glish, G.L.: Tandem mass spectrometry of alkali cationized polysaccharides in a quadrupole ion trap. J. Am. Soc. Mass Spectrom. 8, 987–995 (1997)
Zhang, X., Wang, Y., Hu, L., Guo, D., Fang, X., Zhou, M., Xu, W.: Reducing space charge effects in a linear ion trap by rhombic ion excitation and ejection. J. Am. Soc. Mass Spectrom. 27, 1256–1262 (2016)
Lee, H., Jhang, C.S., Liu, J.T., Lin, C.H.: Rapid screening and determination of designer drugs in saliva by a nib-assisted paper spray-mass spectrometry and separation technique. J. Sep. Sci. 35, 2822–2825 (2012)
Lesiak, A.D., Musah, R.A., Cody, R.B., Domin, M.A., Dane, A.J., Shepard, J.R.: Direct analysis in real time mass spectrometry (DART-MS) of "bath salt" cathinone drug mixtures. Analyst 138, 3424–3432 (2013)
Presley, B.C., Jansen-Varnum, S.A., Logan, B.K.: Analysis of synthetic cannabinoids in botanical material: a review of analytical methods and findings. Forensic Sci. Rev. 25, 27–46 (2013)
Snyder, D.T., Fedick, P.W., Cooks, R.G.: Multigenerational collision-induced dissociation for characterization of organic compounds. Anal. Chem. 88, 9572–9581 (2016)
McLuckey, S.A., Goeringer, D.E.: Slow heating methods in tandem mass spectrometry. J. Mass Spectrom. 32, 461–474 (1997)
Lopez, L.L., Tiller, P.R., Senko, M.W., Schwartz, J.C.: Automated strategies for obtaining standardized collisionally induced dissociation spectra on a benchtop ion trap mass spectrometer. Rapid Commun. Mass Spectrom. 13, 663–668 (1999)
Snyder, D.T., Cooks, R.G.: Multigenerational broadband collision-induced dissociation of precursor ions in a linear ion trap. J. Am. Soc. Mass Spectrom. 27, 1914–1921 (2016)
Snyder, D.T., Cooks, R.G.: Ion isolation and multigenerational collision-induced dissociation using the inverse Mathieu q scan. Rapid Commun. Mass Spectrom. 31, 200–206 (2017)
Evans-Nguyen, T., Becker, L., Doroshenko, V., Cotter, R.J.: Development of a low power, high mass range mass spectrometer for Mars surface analysis. Int. J. Mass Spectrom. 278, 170–177 (2008)
Snyder, D.T., Pulliam, C.J., Wiley, J.S., Duncan, J., Cooks, R.G.: Experimental characterization of secular frequency scanning in ion trap mass spectrometers. J. Am. Soc. Mass Spectrom. 27, 1243–1255 (2016)
Wang, Y., Huang, Z., Jiang, Y., Xiong, X., Deng, Y., Fang, X., Xu, W.: The coupling effects of hexapole and octopole fields in quadrupole ion traps: a theoretical study. J. Mass Spectrom. 48, 937–944 (2013)
Wang, Y., Franzen, J., Wanczek, K.P.: The nonlinear resonance ion trap. Part 2. A general theoretical analysis. Int. J. Mass Spectrom. Ion Processes 124, 125–144 (1993)
Franzen, J.: The nonlinear ion trap. Part 5. Nature of nonlinear resonances and resonant ion ejection. Int. J. Mass Spectrom. Ion Processes 130, 15–40 (1994)
Wang, Y., Franzen, J.: The nonlinear ion trap. Part 3. Multipole components in three types of practical ion trap. Int. J. Mass Spectrom. Ion Processes 132, 155–172 (1994)
Shay, B.J., Eberlin, M.N., Cooks, R.G., Wesdemiotis, C.: Ion-molecule reactions and collision-activated dissociation of C, Hp isomers: a case study in the use of the MS3 capabilities of a pentaquadrupole mass spectrometer. J. Am. Soc. Mass Spectrom. 3, 518–534 (1991)
Sorrilha, A.E., Gozzo, F.C., Pimpim, R.S., Eberlincor, M.N.: Multiple stage pentaquadrupole mass spectrometry for generation and characterization of gas-phase ionic species. The case of the PyC2H 5 (+.) isomers. J. Am. Soc. Mass Spectrom. 7, 1126–1137 (1996)
Juliano, V.F., Gozzo, F.C., Eberlin, M.N., Kascheres, C., do Lago, C.L.: Fast multidimensional (3D and 4D) MS2 and MS3 scans in a high-transmission pentaquadrupole mass spectrometer. Anal. Chem. 68, 1328–1334 (1996)
Snyder, D.T., Pulliam, C.J., Cooks, R.G.: Linear mass scans in quadrupole ion traps using the inverse Mathieu q scan. Rapid Commun. Mass Spectrom. 30, 2369–2378 (2016)
Acknowledgements
The authors acknowledge funding from NASA, Planetary Science Division, Science Mission Directorate (NNX16AJ25G). The authors thank Ryan Hilger (Purdue University) for assistance with the LTQ modifications.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Snyder, D.T., Cooks, R.G. Single Analyzer Precursor Ion Scans in a Linear Quadrupole Ion Trap Using Orthogonal Double Resonance Excitation. J. Am. Soc. Mass Spectrom. 28, 1929–1938 (2017). https://doi.org/10.1007/s13361-017-1707-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-017-1707-y