Abstract
A new atmospheric pressure ionization method termed pyroelectricity-assisted infrared laser desorption ionization (PAI-LDI) was developed in this study. The pyroelectric material served as both sample target plate and enhancing ionization substrate, and an IR laser with wavelength of 1064 nm was employed to realize direct desorption and ionization of the analytes. The mass spectra of various compounds obtained on pyroelectric material were compared with those of other substrates. For the five standard substances tested in this work, LiNbO3 substrate produced the highest ion yield and the signal intensity was about 10 times higher than that when copper was used as substrate. For 1-adamantylamine, as low as 20 pg (132.2 fmol) was successfully detected. The active ingredient in (Compound Paracetamol and 1-Adamantylamine Hydrochloride Capsules), 1-adamantylamine, can be sensitively detected at an amount as low as 150 pg, when the medicine stock solution was diluted with urine. Monosaccharide and oligosaccharides in Allium Cepa L. juice was also successfully identified with PAI-LDI. The method did not require matrix-assisted external high voltage or other extra facility-assisted set-ups for desorption/ionization. This study suggested exciting application prospect of pyroelectric materials in matrix- and electricity-free atmospheric pressure mass spectrometry research.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, infrared laser desorption ionization mass spectrometry (IR-LDI-MS) has achieved rapid development, partly because of its matrix-free feature, where interference peaks from the matrix did not exist. IR-LDI-MS possessed general features such as reduced probability of molecular dissociation and higher neutral-to-ion ratio, because of lower photon energy of infrared (IR) laser in comparison with ultraviolet (UV) laser [1]. In addition, the thermal effect associated with IR lasers can facilitate the desorption of analytes from surfaces [2].
However, IR-LDI usually required extra auxiliary ionization approaches, such as electrospray ionization (ESI) and direct analysis in real time (DART), to improve the ionization efficiency of neutral analytes desorbed from the surface, since low IR energy generally led to low ionization efficiency. For instance, Nemes et al. reported a hybrid ionization method termed laser ablation electrospray ionization (LAESI), which was used to detect verapamil, reserpine and identify certain metabolites in French marigold seeding organs [3]. Rezenom et al. developed an infrared laser-assisted desorption electrospray ionization (IR-LADESI) method for the analysis of peptides and proteins, including bovine cytochrome c, bradykinin, and human whole blood [4]. Another approach, infrared laser desorption electrospray ionization (IR-LDESI) was coupled to LTQ Fourier transform ion cyclotron resonance mass spectrometer, and applied to analyze bovine ubiquitin and myoglobin [5]. Moreover, Shiea’s group reported a laser-induced acoustic desorption/electrospray ionization mass spectrometry (LIAD-ESI-MS) in which the back side of aluminum foil with sample spot was irradiated by IR laser, and the resulting shockwave and heat caused sample desorption from the back side. The desorbed species were ionized with the assistance of ESI plume [6]. Nevertheless, solvent and high voltages were indispensable when ESI was used as the additional ion source.
Plasma was also frequently coupled with LDI-MS methods. For example, Zhang et al. reported that the plasma assisted multi-wavelength (1064, 532, and 355 nm) laser desorption ionization mass spectrometry (PAMLDI-MS) combined with thin layer chromatography (TLC) was applied to analyze low molecular weight compounds [7]. Liu’s group developed surface assisted laser desorption ionization combined with DART to analyze tetradecanoic acid and 4-aminoantipyrine [8]. In addition, high voltage assisted laser desorption ionization mass spectrometry (HALDI-MS) was developed to detect proteins, oligonucleotides, and egg yolk [9]. Although these methods improved ionization efficiency, they required two hybrid devices, laser device and another ionization-assisted setup.
The pyroelectric effect refers to the crystal spontaneous polarization with the change of temperature [10–12]. Present pyroelectric materials have been applied to various fields, including fire alarms, radiometers, pyroelectric vidicon, and X-ray generation etc. [13–15]. LiNbO3 is one of the classic pyroelectric materials, which is often used in IR detectors [16, 17], and LiNbO3 is expected to absorb energy quite uniformly across the IR [18]. Recently, pyroelectric material was applied in dispensing liquids on micrometer- and nanoscale by pyroelectrodynamic shooting. The formation of attolitre liquid droplets was realized by local pyroelectric forces, which were activated by scanning an infrared laser beam over a pyroelectric substrate that was deposited above liquid reservoir [19]. Neidholdt et al. developed a method for detecting triethylamine or triphenylamine through heating pyroelectric crystal to ionize the pre-gasified compounds [10]. Such applications showed the potentials of pyroelectric materials to be utilized in LDI-MS research. It is expected that analytes will undergo direct desorption/ionization by the thermal effect of infrared laser radiation and the shock/vibrating effected produced via pyroelectric material/laser interactions.
In this work, we developed a novel atmospheric pressure ionization method termed pyroelectricity-assisted infrared laser desorption ionization mass spectrometry (PAI-IR-LDI-MS). We employed pyroelectric crystal directly as sample target plate and ionization facility. We compared the ionization capability of pyroelectric material with three other materials (copper, microslide and filter-paper). The results indicated that coupled with infrared laser radiation, the pyroelectric material could be employed as ionization enhancing substrate, and was able to simultaneously desorb and ionize analytes. This study suggested an exciting prospect of pyroelectric materials in ambient mass spectrometry and natural product analysis research.
Experimental
Materials and Chemicals
Z-cut lithium niobate (LiNbO3) was purchased from Shanghai Daheng Optics and Fine Mechanics Co., Ltd. (China). Copper sheets were purchased from Hardware and Electromechanical Supermarket of Warwick Group (China). Quantitative filter paper and microslides were purchased from Peking BioDee Biotechnology Co., Ltd. (China) (Supplementary Table S3). More information about LiNbO3 is shown in Supplementary Table S4.
The chemicals of 1-acetamidoadamantane, 2-aminopyrimidine, spermine, and 2-aminobenzamide were purchased from Alfa Aesar (Heysham, UK). Acetanilide was from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). 1-Adamantylamine was obtained from Sigma-Aldrich (St. Louis, MO, USA). Glucose and Maltose were from Peking BioDee Biotechnology Co., Ltd. (China). Maltotriose was from J&K Scientific Co., Ltd. (Beijing, China). All standard samples were prepared without further treatment (detailed information is shown in Supplementary Tables S1 and S2.). Methanol (HPLC grade) was obtained from Fisher Scientific (Fair Lawn, NJ, USA). Ultrapure water was prepared with Milli-Q (Merck Millipore, Billerica, MA, USA).
The medicine, Compound Paracetanol and Amantadine Hydrochloride Capsules, was purchased from TongRenTang Group Co., Ltd. (Beijing, China). Onion samples (Allium cepa L.) were purchased from a local market.
Ion Source Construction
A home-made ambient LDI ion source was coupled with LTQ Proteome X ion trap mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA) (Figure 1). The ion source consisted of fixed wavelength IR laser system (GYM30-01LY; Beijing Aoyite LLC), and substrates attached to a 3-D translation platform (Beijing Optical Century Instrument Co., Ltd). Nd:YAG combined with pulse xenon lamp were used as pump sources to generate IR pulses of ~10 ns width. The wavelength of laser was 1064 nm and the frequency was 20 Hz. The position of substrate can be manually adjusted. The ion source was operated under atmospheric pressure conditions. Except for the IR-LDI ion source, instead of the standard electrospray source, the mass spectrometer was not otherwise modified.
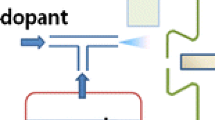
Schematic setup of the IR-LDI ion source and the vertical view of sample spot. Ions were produced at the pyroelectric substrate surface with infrared laser irradiation and travel to the atmospheric pressure orifice capillary of the mass spectrometer. The sample solution was deposited onto the substrate and the laser radiant facula was ~2.0 mm distance from sample spot center
Analyte in the form of solution was deposited onto the substrate and allowed to dry. Sample spot center was a ~6.0-mm distance from MS orifice. The laser irradiated with a ~45° incidence angle to the distal end of MS orifice. The distance from laser incidence to analyte spot center was about 2 mm; however, the laser radiation hit the sample spot. The explanation of parameters selection for the ion source is shown in Support Information.
All mass spectra were collected in positive ion mode with two microscans. Experimental parameters used were as follows: heated capillary temperature, 275.0°C; capillary voltage, 9.0 V; tube lens voltage, 100.0 V; max injection time, 200.0 ms.
Sample Preparation
Amine and amide substances were dissolved in methanol/water according to their dissolution characteristic, and the stock solution was diluted with methanol/water (4/1, v/v) to a series of gradient concentration solutions for further mass spectrometry detection. Saccharides were dissolved by using ultrapure water and then diluted to experimental concentration using methanol/water (1/1, v/v). Sample solution was directly deposited onto the substrate and air-dried, followed immediately by IR laser irradiation for desorption/ionization. The generated ions traveled to mass spectrometer inlet without any extra assisted approach.
Safety Considerations
Eye exposure to laser radiation would cause a severe health hazard. Safety goggles need to be worn while working with free laser beams.
Results and Discussion
Detection of the Analytes Using Various Substrates
In order to investigate the influence of substrate materials on ionization efficiency, four different materials [the pyroelectric material LiNbO3, copper sheet, microslide made of borosilicate glass, and filter paper ([C6H10O5] n)] were tested. Five compounds (1-acetamidoadamantane, acetanilide, 2-aminopyrimidine, spermine, and 2-aminobenzamide) were used for establishing method. The concentration of analyte solution was 100 ppm and the volume dripped on substrate was 1.5–2.0 μL; therefore, the absolute amount of each analyte spot was 150 ng–200 ng. For example, the absolute amount of 1-acetamidoadamantane corresponded to ~775.9 pmol.
The IR-LDI mass spectra of the analytes on different substrates are shown in Figure 2. As can be seen, for all of the five compounds tested, the most abundant MS signals were always obtained from LiNbO3 substrate, and the ratio of S/N arrived at 1 × 104 or so. Ions could also be formed from analytes on copper; however, the abundance of signals decreased a lot. For filter-paper and microslide, the ion signals were very weak or could not be observed at all.
IR-LDI mass spectra of different compounds using different substrates. The samples were: (a) 1-acetamidoadamantane, (b) acetanilide, (c) 2-aminopyrimidine, (d) spermine, (e) 2-aminobenzamide, respectively. The substrates were filter paper, microslide, copper (Cu), and lithium niobate (LiNbO3). The signal abundance was generally 10 times higher on LiNbO3 substrate than other substrates
The mass spectra of 1-acetamidoadamantane on different substrates are shown in Figure 2a. The abundance of [M + H] + (m/z = 194.3) is 11146.2 counts on LiNbO3 substrate, whereas significantly reduced to 879.7, 22.3, and 1.4 on copper, microslide, and filter paper, respectively. Compared with the copper substrate used in the LDI-MS, the sensitivity on LiNbO3 substrate was improved by more than one magnitude. Compared with microslide and filter-paper, the abundances were enhanced by nearly 5.0 × 102 and 7.9 × 103. Dimers of the analyte at [2M + H] + (m/z 387.3) were also detected, which indicated the method of PAI-LDI-MS was a soft ionization mode. Likewise, for the other four compounds, the signal abundances of basic peaks on LiNbO3 substrates were improved by more than 10 times compared with copper substrates, except acetanilide (Figure 2b–e). Moreover, the signal difference among various types of samples was the species of ion. The results showed that aromatic amino (Figure 2e) and aliphatic amino (Figure 2d) substances were more prone to generate [M – NH2]+ signals but heterocyclic amino was not (Figure 2c). Except for the basic peak of [M + H] +, the peak of [M-(CO-CH2) + H] + also generated on LiNbO3 substrate for aromatic amide (Figure 2b).
In all these five standard compounds, the LiNbO3 wafer substrate generated the most distinct MS signals compared with the other three materials. We also examined other pyroelectric materials as substrate like LiTaO3 in preliminary experiments, which also had the efficacy of auxiliary desorption/ionization similar to LiNbO3 (see Supplementary Figure S4). Nevertheless, LiTaO3 is more expensive. Since no organic matrix needed to be used, there was less interference under m / z 500.0, making this method matrix-free and therefore particularly useful in the detection and identification of low molecular weight substances.
The Hypothesis of Desorption/Ionization Mechanism
The desorption/ionization mechanism of PAI-LDI is still not very clear. It is supposed to be attributed to the energy of infrared laser irradiation and shockwave coupled with polarization potential of pyroelectric material. (1) PAI-LDI is one kind of LDI based method, therefore, laser irradiation probably contributes to the desorption/ionization [20]. (2) The interaction between laser and substrate, like discharging and shockwave, could contribute to the desorption/ionization. We observed sparkling/discharging when LiNbO3 was irradiated by laser. The m/z 279.2 (see Figure 1a) was one of the major background peaks, which was suspected to the protonated dibutyl phthalate from laboratory air. The m/z 149.1 (Figure 3) was probably one characteristic fragment of the substances of phthalate. The result was consistent with Neidholdt and Beauchamp’s study [21, 22]. For analyte desorption, we speculate that shockwave and thermal effect [2, 23] were simultaneously responsible, leading to the liberation of analytes from the pyroelectric material substrate. We suppose that the shockwave is generated by the interaction of laser and LiNbO3 interaction. (3) The pyroelectric effect could contribute to the desorption/ionization. We preliminarily measured the temperature change before/after laser irradiation onto LiNbO3 substrate. The pyroelectric potential probably has the function of ionization-assisted [10]. The ionization mechanism of PAI-LDI is probably attributable to all the factors simultaneously in action. However, the mechanism of ionization was complicated. Additionally, we compared PAI-LDI with other IR-LDI methods cited in the Introduction section, and the table is shown in Supplementary Table S5.
Analysis of Mixtures Using PAI-LDI-MS
To evaluate the potentials of PAI-LDI-MS in the analysis of complex mixtures, a drop of 1.5 μL mixture of solution containing 2-aminopyrimidine, 1-acetamidoadamantane, and acetanilide was deposited onto LiNbO3 substrate. The amount of each component was 150.0 ng within the sample spot formed. As can be seen from Figure 3, all analytes can be readily detected. By contrast, when copper sheet was used as the substrate, the MS signal abundances were significantly lowered by 3.00 (m / z 96.1), 9.86 (m / z 194.3), 12.52 (m / z 136.2), and 13.11 (m / z 387.3) times, respectively. Almost nothing can be detected from microslides and filter paper (see Supplementary Figure S1).
It seemed that the response of analytes in the mixture of three standards appears to be different than the PAI-LDI response to the individual analytes. This may be associated with matrix effects. In our experiment, no specific matrix was used to assist ionization; therefore, there was no matrix interference introduced by experiment design. However, for the complex system, such as mixed sample, the matrix effect probably was unavoidable. In this case, isotope internal standard method would be useful for precise quantification [24].
Concentration Dependence, Precision, and Reproducibility
The standard substance of 1-adamantylamine (amantadine) was dissolved in methanol/water (v/v, 1:1), and was diluted to five different gradient concentrations with100, 10, 1 ppm, 100 ppb, and 10 ppb. Sample volumes of 2 μL (corresponding to 200, 20, 2 ng, 200 pg, and 20 pg, respectively) were deposited on the LiNbO3 substrate for MS detection. Amantadine was successfully detected on LiNbO3 substrate at as low as 20 pg (132.2 fmol) (Figure 4a). Considering the estimates of analyte amounts in the area interrogated by laser pulse, for laser-based methods, the limit of detection could be even lower [3] (see Support Information). A linear relationship between the analyte amount and signal response can be obtained after taking logarithm for both variables (Figure 4b).
Concentration dependence, precision, and reproducibility of PAI-LDI-MS method. (a) PAI-LDI mass spectra of 1-adamantylamine with different concentration gradient. The substance content of 1-adamantylamine in each sample point was 200, 20, 2, 200, 20 pg (corresponding to 132.2 fmol), respectively. (b) Linear relationship between the analyte amount and signal response after taking logarithm for both variables. (c) Precision. (d) Reproducibility
The precision of this ionization method was evaluated on the same LiNbO3 wafers. The solution of 1-acetamidoadamantane at a concentration of 100 ppm was prepared; 1.5 μL 1-acetamidoadamantane solution with was deposited on one LiNbO3 wafer for mass spectrometry. The same experimental procedures were repeated three times on one same LiNbO3 wafer. The mass spectra obtained are shown in Figure 4c. The relative standard deviation (RSD) of the intensity of the analyte as [M + H]+ was 1.67%, which indicated the precision of this ionization method was fine.
The reproducibility experiment on five different LiNbO3 wafers was also investigated through the above same manipulation repeated five times. The result is shown in Figure 4d. RSD for protonated ion [M + H] + was 10.52%, and coefficient of correlation was 1.0 for five mass spectra, indicating that although signal abundance varied, the total signal status nevertheless was very stable among separate experiments. Additionally, the signal deviation was probably due to the LiNbO3 wafers from different production batches and the atmospheric pressure conditions.
Analysis of Real Samples
Amantadine (1-adamantylamine) is one of the major active ingredients in the medicine of Compound Paracetamol and Amantadine Hydrochloride Capsules. It was the classic prophylactic agent against Asian influenza and drug for treatment of influenza A virus [25, 26]. Amantadine has become part of the arsenal utilized for early symptomatic treatment of Parkinson’s disease, as well as an option for treating dyskinesia [27–29]. In our study, the medicine capsules were first dissolved in ultrapure water, and the supernatant liquid was taken as stock solution for the subsequent experiments.
The stock solution was diluted to 10 ppm and 10 ppb using methanol/water, which was then deposited onto different substrates for mass spectrometry analysis. Mass spectra obtained are shown in Figure 5. The signal of 1-adamantylamine was clearly visible in the form of [M + H]+ (m/z 152.2) when LiNbO3 was used as substrate, at a concentration of as low as 10 ppb. At the concentration of 10 ppm, the intensity ratio between [M + H]+ to a background peak at m/z 158.2 was ~5.0. When copper was used as the substrate, the same ratio became lower than 1.0. This indicated that PAI-LDI-MS could identify active ingredients from medicines.
Amantadine is also used for the treatment of hepatitis C and multiple sclerosis. As reported, amantadine is metabolized mainly through urine pathway, and it is nearly completely eliminated in the urine, largely unchanged [30, 31]. Up to now, the detection or analysis of amantadine commonly uses the traditional method, which is gas chromatography [32] or fluorimetric liquid chromatography [33]. It has not been reported that amantadine was detected by ambient mass spectrometry. To explore the potentials of PAI-LDI-MS method for analyzing medicine metabolism in a sample preparation-free approach, the stock solution of medicine was diluted with human urine to 100, 1 ppm, and 100 ppb. The MS signal of the amantadine, the active ingredient of the medicine, can be clearly observed at 100 ppb concentration in urine (~150 ng, see Figure 6). It seemed that 1-adamantylamine (amantadine), the active ingredient of medicine capsules, was determined by PAI-LDI down to a concentration of 10 ppb for the medicine dissolved in methanol/water (Figure 5), whereas 100 ppb for the medicine dissolved in urine (Figure 6), which was possibly caused by much complex matrix effects.
There was a report that the urinary excretion concentration of amantadine was ~50–200 μg/mL (corresponding to 50 ppm–200 ppm) [34]. In our experiments, the simulated detection concentration of amantadine in urine, from ~100 ppb to 100 ppm, was basically consistent with the above reports. This showed PAI-LDI has the potential for analyzing medicine metabolism. The mass spectrum (multi-point average) of urine sample showing the entire mass range, including spiked 1-adamantylamine and urine native creatinine [35], is shown in Supplementary Figure S3. The result shows that the components of urine have little influence on analyte signal. This indicated that the method of PAI-LDI has a high tolerance to complex matrix. Our result demonstrated that the developed method can be deployed for the confident and direct identification of small molecules from complex samples.
PAI-LDI-MS of the method was also used to directly analyze the composition of Allium Cepa L. by depositing onion juice onto LiNbO3 wafer. Three components, including monosaccharide, disaccharide, and trisaccharide, were identified at [M1]+ (m/z = 180.1), [M1 + NH4]+ (m/z = 198.1), [M2]+ (m/z = 342.2), [M3]+ (m/z = 504.1), respectively. The result showed that for the analysis of oligosaccharide with the method of PAI-LDI-MS, [M]+ was more easily generated. The mass spectra are shown in Figure 7. The result, from another aspect, clearly shows the capability of the developed method for the analysis of biological system. The PAI-LDI mass spectra of these standard saccharide compounds are shown in Supplementary Figure S2.
Conclusions
In this study, we developed a new atmospheric pressure ionization method termed pyroelectricity-assisted infrared laser desorption mass spectrometry (PAI-LDI-MS).The pyroelectric material LiNbO3, which was used as the substrate but at the same time facilitated analytes desorption/ionization, led to significantly abundant analyte signal than other common substrate materials. Due to its matrix-free nature, the developed method is particularly useful for the direct detection of low molecular weight compounds without interference from the background. 1-Adamantylamine was successfully detected at a concentration as low as 10 ppb (corresponds to 132.2 fmol). It is also tolerant to complex matrix, and active medicine ingredient in urine can be detected. The method was also applied to analyze complex samples, such as Allium Cepa L. from which saccharide components were detected. Overall, the advantageous features of the developed method include no requirement for high voltage and matrix, simple pretreatment, and soft ionization capability. It is expected that such a method will find wide applications in the study of medicine metabolism by analyzing human body fluids and fast analysis of complex real-world samples.
References
Dreisewerd, K.: The desorption process in MALDI. Chem. Rev. 103, 395–425 (2003)
Little, M.: Infrared laser desorption/ionization mass spectrometry: fundamentals and applications. Dissertation, Louisiana State University (2006)
Nemes, P., Vertes, A.: Laser ablation electrospary ionization for atmospheric pressure, in vivo, and imageing mass spectrometry. Anal. Chem. 79, 8098–8106 (2007)
Rezenom, Y.H., Dong, J., Murray, K.K.: Infrared laser-assisted desorption electrospray ionization mass spectrometry. Analyst 133, 226–232 (2008)
Sampson, J.S., Muddiman, D.C.: Atmospheric pressure infrared (10.6 microm) laser desorption electrospray ionization (IR-LDESI) coupled to a LTQ Fourier transform ion cyclotron resonance mass spectrometer. Rapid Commun. Mass Spectrom. 23, 1989–1992 (2009)
Cheng, S.-C., Cheng, T.-L., Chang, H.-C., Shiea, J.: Using laser-induced acoustic desorption/electrospray ionization mass spectrometry to characterize small organic and large biological compounds in the solid state and in solution under ambient conditions. Anal. Chem. 81, 868–874 (2009)
Zhang, J., Zhou, Z., Yang, J., Zhang, W., Bai, Y., Liu, H.: Thin layer chromatography/plasma assisted multiwavelength laser desorption ionization mass spectrometry for facile separation and selective identification of low molecular weight compounds. Anal. Chem. 84, 1496–1503 (2012)
Zhang, J., Li, Z., Zhang, C., Feng, B., Zhou, Z., Bai, Y., Liu, H.: Graphite-coated paper as substrate for high sensitivity analysis in ambient surface-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 84, 3296–3301 (2012)
Ren, X., Liu, J., Zhang, C., Luo, H.: Direct analysis of samples under ambient condition by high-voltage-assisted laser desorption ionization mass spectrometry in both positive and negative ion mode. Rapid Commun. Mass Spectrom. 27, 613–620 (2013)
Neidholdt, E.L., Beauchamp, J.L.: Compact ambient pressure pyroelectric ion source for mass spectrometry. Anal. Chem. 79, 3945–3948 (2007)
Lang, S.B.: Pyroelectricity: from ancient curiosity to modern imaging tool. Phys. Today 58, 31–36 (2005)
Yun, Y., Kampschulte, L., Li, M., Liao, D., Altman, E.I.: Effect of ferroelectric poling on the adsorption of 2-propanol on LiNbO3(0001). J. Phys. Chem. C 111, 13951–13956 (2007)
Whatmore, R.W.: Pyroelectric devices and materials. Rep. Prog. Phys. 49, 1335–1386 (1986)
Geuther, J.A., Danon, Y.: High-energy X-ray production with pyroelectric crystals. J. Appl. Phys. 97, 104916 (2005)
Hadni, A.: Applications of the pyroelectric effect. J. Phys. E Sci Instrum 14, 1233–1240 (1981)
Gebre, T., Batra, A.K., Guggilla, P., Aggarwal, M.D., Lal, R.B.: Pyroelectric properties of pure and doped lithium niobate crystals for infrared sensors. Ferroelectr. Lett. 31, 131–139 (2004)
Muralt, P.: Micromachined infrared detectors based on pyroelectric thin films. Rep. Prog. Phys. 64, 1339–1388 (2001)
Muir, A.C.: Interactions of single-crystal lithium niobate surfaces with ultra-violet laser radiation. Dissertation, England. University of Southampton, pp. 7–12 (2008)
Ferraro, P., Coppola, S., Grilli, S., Paturzo, M., Vespini, V.: Dispensing nano-pico droplets and liquid patterning by pyroelectrodynamic shooting. Nat. Nanotechnol. 5, 429–435 (2010)
de Hoffmann, E., Stroobant, V.: Mass Spectrometry Principles and Applications. Wiley, Chichester, pp. 32–34 (2007)
Neidholdt, E.L., Beauchamp, J.L.: Ionization mechanism of the ambient pressure pyroelectric ion source (APPIS) and its applications to chemical nerve agent detection. J. Am. Soc. Mass Spectrom. 20, 2093–2099 (2009)
Neidholdt, E.L., Beauchamp, J.L.: Switched ferroelectric plasma ionizer (SwiFerr) for ambient mass spectrometry. Anal. Chem. 83, 38–43 (2011)
Rockwell, D.A., Parks, J.H.: Theory of acoustic-surface-wave detection of radiative absorption. J. Appl. Phys. 47, 2889 (1976)
Espy, R.D., Teunissen, S.F., Manicke, N.E., Ren, Y., Ouyang, Z., van Asten, A., Cooks, R.G.: Paper spray and extraction spray mass spectrometry for the direct and simultaneous quantification of eight drugs of abuse in whole blood. Anal. Chem. 86, 7712–7718 (2014)
Maugh, T.: Amantadine: an alternative for prevention of influenza. Science 192, 130–131 (1976)
Alves Galvao, M.G., Rocha Crispino Santos, M.A., Alves da Cunha, A.J.: Amantadine and rimantadine for influenza A in children and the elderly. Cochrane Database Syst. Rev. 1, 1–120 (2012)
Hubsher, G., Haider, M., Okun, M.S.: Amantadine— the journey from fighting flu to treating Parkinson disease. Neurology 78, 1096–1099 (2012)
Tribl, G.G., Wober, C., Schonborn, V., Brucke, T., Deecke, L., Panzer, S.: Amantadine in Parkinson’s disease: lymphocyte subsets and IL-2 secreting T cell precursor frequencies. Exp. Gerontol. 36, 1761–1771 (2001)
Relja, M., Bozikov, J.: De-novo amantadine treatment prevents and delays onset of dyskinesias in Parkinson’s disease. Mov. Disord. 29, S146 (2014)
Koppel, C., Tenczer, J.: A revision of the metabolic disposition of amantadine. Biomed. Mass Spectrom. 12, 499–501 (1985)
Farajzadeh, M.A., Nouri, N., Alizadeh Nabil, A.A.: Determination of amantadine in biological fluids using simultaneous derivatization and dispersive liquid–liquid microextraction followed by gas chromatography-flame ionization detection. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 940, 142–149 (2013)
Stumph, M.J., Noall, M.W., Knight, V.: Gas-chromatographic determination of amantadine in human-urine. Clin. Chem. 26, 295–296 (1980)
Duh, T.H., Wu, H.L., Pan, C.W., Kou, H.S.: Fluorimetric liquid chromatographic analysis of amantadine in urine and pharmaceutical formulation. J. Chromatogr. A 1088, 175–181 (2005)
Montanari, C., Ferrari, P., Bavazzano, A.: Urinary excretion of amantadine by the elderly. Eur. J. Clin. Pharmacol. 8, 349–351 (1975)
Chen, S., Chen, L., Wang, J., Hou, J., He, Q., Liu, J., Wang, J., Xiong, S., Yang, G., Nie, Z.: 2,3,4,5-Tetrakis(3′,4′-dihydroxylphenyl)thiophene: a new matrix for the selective analysis of low molecular weight amines and direct determination of creatinine in urine by MALDI-TOF MS. Anal. Chem. 84, 10291–10297 (2012)
Acknowledgments
The authors acknowledge support for this work by the Ministry of Science and Technology of China (no. 2013CB933800 and 2012YQ12006003), and the National Natural Science Foundation of China (no. 21390411 and 21125525).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 467 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Ma, X., Wei, Z. et al. Pyroelectricity Assisted Infrared-Laser Desorption Ionization (PAI-LDI) for Atmospheric Pressure Mass Spectrometry. J. Am. Soc. Mass Spectrom. 26, 1266–1273 (2015). https://doi.org/10.1007/s13361-015-1154-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-015-1154-6