Abstract
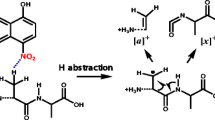
Two novel monofunctionalized fulleropyrrolidine derivatives (Prato adducts) were prepared and characterized by matrix-assisted laser desorption/ionization (MALDI) and electrospray ionization (ESI). MALDI experiments conducted in the positive-ion mode on pure and mixed samples of both monofunctionalized fullerene derivatives revealed the efficient formation of bisadducts (in the case of the pure samples) and mixed bisadducts (in the case of a mixed sample). Bisadducts were not observed in the ESI experiments and thus not present in the sample. A mechanism for the MALDI formation of these bisadduct ions is proposed in which an azomethine ylide fragment is formed in situ from the monofunctionalized fulleropyrrolidine species upon laser irradiation. This fragment, which can survive as an intact moiety in the gas phase in the special environment provided by the MALDI experiment, is then able to attach to a fulleropyrrolidine monoadduct which acts as a dipolarophile, thus leading to the formation of a bisadduct fullerene derivative. The unprecedented re-attachment of the azomethine ylide implies that the establishment of the ligand attainment of Prato adducts based on MALDI analysis alone can lead to wrong assignments.

ᅟ






Similar content being viewed by others
References
Barrow, M.P., Feng, X., Wallace, J.I., Boltalina, O.V., Taylor, R., Derrick, P.J., Drewello, T.: Characterization of fullerenes and fullerene derivatives by nanospray. Chem. Phys. Lett. 330, 267–274 (2000)
Kozlovski, V., Brusov, V., Sulimenkov, I., Pikhtelev, A., Dodonov, A.: Novel experimental arrangement developed for direct fullerene analysis by electrospray time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 18, 780–786 (2004)
Greisch, J.F., De Pauw, E.: Mass spectrometric characterization of 3′-imino[60]fulleryl-3′-deoxythymidine by collision-induced dissociation. J. Mass Spectrom. 42, 304–311 (2007)
Knochenmuss, R., Zenobi, R.: MALDI ionization: the role of in-plume processes. Chem. Rev. 103, 441–452 (2003)
Fati, D., Leeman, V., Vasil’ev, Y.V., Drewello, T., Leyh, B., Hungerbühler, H.: Alkali cation attachment to derivatized fullerenes studied by matrix-assisted laser desorption/ionization. J. Am. Soc. Mass Spectrom. 13, 1448–1458 (2002)
Fati, D., Vasil’ev, Y.V., Wachter, N.K., Taylor, R., Drewello, T.: MALDI mass spectrometric determination of molecular site-specific charge localization, and laser-induced coalescence reactivity of fullerenodendrimers. Int. J. Mass Spectrom. 229, 3–10 (2003)
Vasil'ev, Y.V., Khvostenko, O.G., Streletskii, A.V., Boltalina, O.V., Kotsiris, S.G., Drewello, T.: Electron transfer reactivity in matrix-assisted laser desorption/ionization (MALDI): ionization energy, electron affinity and performance of the DCTB matrix within the thermochemical framework. J. Phys. Chem. A 110, 5967–5972 (2006)
Zhou, L., Deng, H., Deng, Q., Zheng, L., Cao, Y.: Analysis of three different types of fullerene derivatives by laser desorption/ionization time-of-flight mass spectrometry with new matrices. Rapid Commun. Mass Spectrom. 19, 3523–3530 (2005)
Hirsch, A., Brettreich, M.: The Chemistry of Fullerenes. Wiley-VCH, Weinheim, Germany (2005). pp. 141–151
Martín, N., Altable, M., Filippone, S., Martín-Domenech, A.: New reactions in fullerene chemistry. Synlett 3077–3095 (2007)
Maggini, M., Scorrano, G., Prato, M.: Addition of azomethine ylides to C60: synthesis, characterization, and functionalization of fullerene pyrrolidines. J. Am. Chem. Soc. 115, 9798–9799 (1993)
Prato, M., Maggini, M.: Fulleropyrrolidines: a family of full-fledged fullerene derivatives. Acc. Chem. Res. 31, 519–526 (1998)
Guldi, D.M., Martín, N. (eds.): Fullerenes: From Synthesis to Optoelectronic Properties. Kluwer Academic, Dordrecht, The Netherlands (2002)
Langa, F., Nierengarten, J.-F. (eds.): Fullerenes: Principles and Applications. Nanoscience and Nanotechnology Series. The Royal Society of Chemistry, Cambridge, UK (2007)
Martin, N., Altable, M., Filippone, S., Martin-Domenech, A., Echegoyen, L., Cardona, C.M.: Retro-cycloaddition reaction of pyrrolidinofullerenes. Angew. Chem. Int. Ed. 45, 110–104 (2006)
Lukoyanova, O., Cardona, C.M., Altable, M., Filippone, S., Domenech, A.M., Martin, N., Echegoyen, L.: Selective electrochemical retro-cycloaddition reaction of pyrrolidinofullerenes. Angew. Chem. Int. Ed. 45, 7430–7433 (2006)
Filippone, S., Barroso, M.I., Martin-Domenech, A., Osuna, S., Sola, M., Martin, N.: On the mechanism of the thermal retrocycloaddition of pyrrolidinofullerenes (retro-Prato reaction). Chem. Eur. J. 14, 5198–5206 (2008)
Delgado, J., Filippone, S., Martín-Domenech, A., Altable, M., Maroto, E., Langa, F., Martín, N., Martínez-Alvarez, R.: Mass spectrometry studies of the retro-cycloaddition reaction of pyrrolidino and 2-pyrazolinofullerene derivatives under negative ESI conditions. J. Am. Soc. Mass Spectrom. 22, 557–567 (2011)
Maroto, E., Filipone, S., Martin-Domenech, A., Suarez, M., Martin, N., Martinez-Alvarez, R.: Effect of substituents and protonation on the mechanism of 1,3-dipolar retro-cycloaddition reaction of pyrrolidino[60]- and [70] fullerenes. J. Mass Spectrom. 46, 1016–1029 (2011)
Delgado, J.L., Osuna, S., Bouit, P.-A., Martínez-Alvarez, R., Espíldora, E., Solà, M., Martín, N.: Competitive retro-cycloaddition reaction in fullerene dimers connected through pyrrolidinopyrazolino rings. J. Org. Chem. 74, 8174–8180 (2009)
Ulmer, L., Mattay, J., Torres-Garcia, H.G., Luftmann, H.: The use of 2-[(2E)-3-(4-tert-butylphenyl)-2-methylprop-2- enylidene]malononitrile as a matrix for matrix-assisted laser desorption/ionization mass spectrometry. Eur. J. Mass Spectrom. 6, 49–52 (2000)
Ramos, C.I.V., Santana-Marques, M.G., Enes, R.F., Tomé, A.C., Cavaleiro, J.A.S., Nogueras, M.: Gas-phase fragmentation of protonated C60-pyrimidine derivatives. J. Mass Spectrom. 44, 911–919 (2009)
Kuvychko, I.V., Streletskii, A.V., Popov, A.A., Kotsiris, S.G., Drewello, T., Strauss, S.H., Boltalina, O.V.: Seven-minute synthesis of pure CS-C60Cl6 from [60]fullerene and iodine monochloride: first IR, Raman, and mass spectra of 99 mol % C60Cl6. Chem. Eur. J. 11, 5426–5436 (2005)
Streletskii, A.V., Ioffe, I.N., Kotsiris, S.G., Barrow, M.P., Drewello, T., Strauss, S.H., Boltalina, O.V.: In-plume thermodynamics of the MALDI generation of fluorofullerene anions. J. Phys. Chem. A 109, 714–719 (2005)
Beck, R.D., Stroemer, C., Schulz, C., Michel, R., Weis, P., Bräuchle, G., Kappes, M.M.: Enhanced coalescence upon laser desorption of fullerene oxides. J. Chem. Phys. 101, 3243–3249 (1994)
Beulen, M.W.J., Rivera, J.A., Herranz, M.A., Martin-Domenech, A., Martin, N., Echegoyen, L.: Reductive electrolysis of [60]fullerene mono-methanoadducts in THF leads to the formation of bis-adducts in high yields. Chem. Commun 407–408 (2001)
Acknowledgments
The authors thank Maite Alonso Pascual and Mª Jesus Vicente Arana from the SIdI of the Universidad Autónoma de Madrid, Spain, for recording some of the mass spectra. Support is acknowledged from the Spanish MICINN (CTQ2011-24187/BQU and CONSOLIDER INGENIO 2010, CSD2007-00010 on Molecular Nanoscience) and the CAM (MADRISOLAR-2, S2009/PPQ/ 1533). The authors thank the German Science Foundation (DFG)–SFB 953 Synthetic Carbon Allotropes–for their financial support.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 3751 kb)
Rights and permissions
About this article
Cite this article
Bottari, G., Dammann, C., Torres, T. et al. Laser-Induced Azomethine Ylide Formation and Its Covalent Entrapment by Fulleropyrrolidine Derivatives During MALDI Analysis. J. Am. Soc. Mass Spectrom. 24, 1413–1419 (2013). https://doi.org/10.1007/s13361-013-0680-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-013-0680-3