Abstract
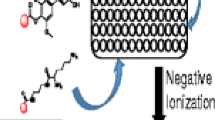
Analyte-matrix adducts are normally absent under typical matrix assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI TOF MS) conditions. Interestingly, though, in the analysis of several types of organic compounds synthesized in our laboratory, analyte-matrix adduct ion peaks were always recorded when common MALDI matrices such as 4-hydroxy-α-cyanocinnamic acid (CHCA) were used. These compounds are mainly those with a benzene-1,3,5-tricarboxamide (BTA) or urea moiety, which are important building blocks to make new functional supramolecular materials. The possible mechanism of the adduct formation was investigated. A shared feature of the compounds studied is that they can form intermolecular hydrogen bonding with matrices like CHCA. The intermolecular hydrogen bonding will make the association between analyte ions and matrix molecules stronger. As a result, the analyte ions and matrix molecules in MALDI clusters will become more difficult to be separated from each other. Furthermore, it was found that analyte ions were mainly adducted with matrix salts, which is probably due to the much lower volatility of the salts compared with that of their corresponding matrix acids. It seems that the analyte-matrix adduct formation for our compounds are caused by the incomplete evaporation of matrix molecules from the MALDI clusters because of the combined effects of enhanced intermolecular interaction between analyte-matrix and of the low volatility of matrix salts. Based on these findings, strategies to suppress the analyte-matrix adduction are briefly discussed. In return, the positive results of using these strategies support the proposed mechanism of the analyte-matrix adduct formation.

ᅟ



Similar content being viewed by others
References
Roosma, J., Mes, T., Leclere, P.E.L.G., Palmans, A.R.A., Meijer, E.W.: Supramolecular materials from benzene-1,3,5-tricarboxamide-based nanorods. J. Am. Chem. Soc. 130, 1120–1121 (2008)
Mes, T., van der Weegen, R., Palmans, A.R.A., Meijer, E.W.: Single-chain polymeric nanoparticles by stepwise folding. Angew. Chem. Int. Ed. 50, 5085–5089 (2011)
Cantekin, S., Balkenende, D.W.R., Smulders, M.M.J., Palmans, A.R.A., Meijer, E.W.: The effect of isotopic substitution on the chirality of a self-assembled helix. Nat. Chem. 3, 42–46 (2011)
Pal, A., Karthikeyan, S., Sijbesma, R.P.: Coexisting hydrophobic compartments through self-sorting in rod-like micelles of bisurea bolaamphiphiles. J. Am. Chem. Soc. 132, 7842–7843 (2010)
Versteegen, R.M., Sijbesma, R.P., Meijer, E.W.: Synthesis and characterization of segmented copoly(ether urea)s with uniform hard segments. Macromolecules 38, 3176–3184 (2005)
Dankers, P.Y.W., Harmens, M.C., Brouwer, L.A., van Luyn, M.J.A., Meijer, E.W.: A modular and supramolecular approach to bioactive scaffolds for tissue engineering. Nat. Mat. 4, 568–574 (2005)
de Greef, T.F.A., Smulders, M.M.J., Wolffs, M., Schenning, A.P.H.J., Meijer, E.W.: Supramolecular polymerization. Chem. Rev. 109, 5687–5754 (2009)
Stals, P.J.M., Korevaar, P.A., Gillissen, M.A.J., de Greef, T.F.A., Fitie, C.F.C., Sijbesma, R.P., Palmans, A.R.A., Meijer, E.W.: Symmetry breaking in the self-assembly of benzene-1,3,5-tricarboxamide. Angew. Chem. Int. Ed. 51, 11297–11301 (2012)
Aida, T., Meijer, E.W., Stupp, S.I.: Functional supramolecular polymers. Science 335, 813–817 (2012)
Fagerquist, C.K., Sultan, O., Carter, M.Q.: Possible evidence of amide bond formation between sinapinic acid and lysine-containing bacterial proteins by matrix –assisted laser desorption/ionization (MALDI) at 355 nm. J. Am. Soc. Mass Spectrom. 23, 2102–2114 (2012)
Beavis, R.C., Chait, B.T.: High-accuracy molecular mass determination of proteins using matrix-assisted laser desorption mass spectrometry. Anal. Chem. 62, 1836–1840 (1990)
Lou, X., de Waal, B.F.M., van Dongen, J.L.J., Vekemans, J.A.J.M., Meijer, E.W.: A pitfall of using 2-[(2E)-3-(4-tert-butylphenyl)-2-methylprop-2-enylidene]malononitrile as a matrix in MALDI TOF MS: chemical adduction of matrix to analyte amino groups. J. Mass Spectrom. 45, 1195–1202 (2010)
Loboda, A.V., Chernushevich, I.V.: Investigation of the mechanism of matrix adduct formation in MALDI at elevated pressure. Int. J. Mass Spectrom. 240, 101–105 (2005)
Keller, B.O., Li, L.: Discerning matrix-cluster peaks in matrix-assisted laser desorption/ionization time-of-flight mass spectra of dilute peptide mixtures. J. Am. Soc Mass Spectrom. 11, 88–93 (2000)
Kim, J.S., Kim, J.Y., Kim, H.J.: Suppression of matrix clusters and enhancement of peptide signals in MALDI TOF mass spectrometry using nitrilotriacetic acid. Anal. Chem. 77, 7483–7488 (2005)
Smirnov, I.P., Zhu, X., Taylor, T., Huang, Y., Ross, P., Papayanopoulos, I.A., Martin, S.A., Pappin, D.J.: Suppression of α-cyano-4-hydroxycinnamic acid matrix clusters and reduction of chemical noise in MALDI-TOF mass spectrometry. Anal. Chem. 76, 2958–2965 (2004)
Guo, Z., Zhang, Q., Zou, H., Guo, B., Ni, J.: A method for the analysis of low-mass molecules by MALDI-TOF mass spectrometry. Anal. Chem. 74, 1637–1641 (2002)
Harris, W.A., Janecki, D.J., Reilly, J.P.: Use of matrix clusters and trypsin autolysis fragments as mass calibrants in matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 16, 1714–1722 (2002)
Billeci, T.M., Stults, J.T.: Tryptic mapping of recombinant proteins by matrix-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 65, 1709–1716 (1993)
Stals, P.J.M., Haveman, J.F., Martin-Rapun, R., Fitié, C.F.C., Palmans, A.R.A., Meijer, E.W.: The influence of oligo(ethylene glycol) side chains on the self-assembly of benzene-1,3,5-tricarboxamides in the solid state and in solution. J. Mater. Chem. 19, 124–130 (2009)
Wisse, E., Govaert, L.E., Meijer, H.E.H., Meijer, E.W.: Unusual tuning of mechanical properties of thermoplastic elastomers using supramolecular fillers. Macromolecules 39, 7425–7432 (2006)
Ulmer, L., Mattay, J., Torres-Garcia, H.G., Luftmann, H.: The use of 2-[(2E)-3-(4-tert-butylphenyl)-2-methylprop-2-enylidene]malononitrile as matrix for matrix-assisted laser desorption/ionization mass spectrometry. Eur. J. Mass Spectrom. 6, 49–52 (2000)
Langeveld-Voss, B.M.W.: Ph.D. thesis, Eindhoven University of Technology, 1999
Karas, M., Kruger, R.: Ion formation in MALDI: cluster ionization mechanism. Chem. Rev. 103, 427–439 (2003)
Karas, M., Gluckmann, M., Schafer, J.: Ionization in matrix-assisted laser desorption/ionization: singly charged molecular ions are the lucky survivors. J. Mass Spectrom. 35, 1–12 (2000)
Farmer, T.B., Caprioli, R.M.: Determination of protein-protein interactions by matrix-assisted laser desorption/ionization mass spectrometry. J. Mass Spectrom. 33, 697–704 (1998)
Cheng, S.W., Chan, T.W.D.: Use of ammonium halides as co-matrices for matrix-assisted laser desorption/ionization studies of oligonucleotides. Rapid Commun. Mass Spectrom. 10, 907–910 (1996)
Asara, J.M., Allison, J.: Enhanced detection of oligonucleotides in UV MALDI MS using tetraamine spermine as matrix additive. Anal. Chem. 71, 2866–2870 (1999)
Acknowledgment
The authors thank Dr. C.F.C Fitié and Dr. E. Wisse for kindly providing BTA-1PEG (A2) and Urea2 (A6) samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lou, X., Fransen, M., Stals, P.J.M. et al. Unusual Analyte-Matrix Adduct Ions and Mechanism of Their Formation in MALDI TOF MS of Benzene-1,3,5-Tricarboxamide and Urea Compounds. J. Am. Soc. Mass Spectrom. 24, 1405–1412 (2013). https://doi.org/10.1007/s13361-013-0672-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-013-0672-3