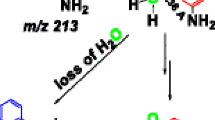
Abstract
In addition to the well-known SO2 loss, there are several additional fragmentation pathways that gas-phase anions derived from N-phenyl benzenesulfonamides and its derivatives undergo upon collisional activation. For example, N-phenyl benzenesulfonamide fragments to form an anilide anion (m/z 92) by a mechanism in which a hydrogen atom from the ortho position of the benzenesulfonamide moiety is specifically transferred to the charge center. Moreover, after the initial SO2 elimination, the product ion formed undergoes primarily, an inter-annular H2 loss to form a carbazolide anion (m/z 166) because the competing intra-annular H2 loss is significantly less energetically favorable. Results from tandem mass spectrometric experiments conducted with deuterium-labeled compounds confirmed that the inter-ring mechanism is the preferred pathway. Furthermore, N-phenyl benzenesulfonamide and its derivatives also undergo a phenyl radical loss to form a radical ion with a mass-to-charge ratio of 155, which is in violation of the so-called “even-electron rule.”

ᅟ










Similar content being viewed by others
References
Anand, N.: Sulfonamides and Sulfones. In: Wolff, M.E. (ed.) Burger’s Medicinal Chemistry, 4th edn, pp. 1–40. Wiley, Chichester (1979)
Kang, J.G., Hur, J.H., Choi, S.J., Choi, G.J., Cho, K.Y., Ten, L.N., Park, K.H., Kang, K.Y.: Antifungal activities of N-arylbenzenesulfonamides against phytopathogens and control efficacy on wheat leaf rust and cabbage club root diseases. Biosci. Biotechnol. Biochem. 66, 2677–2682 (2002)
Li, J.J., Wang, H., Tino, J.A., Robl, J.A., Herpin, T.F., Lawrence, R.M., Biller, S., Jamil, H., Ponticiello, R., Chen, L., Chu, C.-H., Flynn, N., Cheng, D., Zhao, R., Chen, B., Schnur, D., Obermeier, M.T., Sasseville, V., Padmanabha, R., Pike, K., Harrity, T.: 2-Hydroxy-N-arylbenzenesulfonamides as ATP-citrate lyase inhibitors. Bioorg. Med. Chem. Lett. 17, 3208–3211 (2007)
Saíz-Urra, L., González, M.P., Collado, I.G., Hemández-Galán, R.: Quantitative structure–activity relationship studies for the prediction of antifungal activity of N-arylbenzenesulfonamides against Botrytis cinerea. J. Mol. Graph. Model. 25, 680–690 (2007)
Stowe, C.M.: The sulfonamides. In: Jones, L.M. (ed.) Veterinary Pharmacology and Therapeutics, pp. 438–502. Iowa State University Press, Ames (1965)
Badoil, L., Benanou, D.: Characterization of volatile and semivolatile compounds in waste landfill leachates using stir bar sorptive extraction-GC/MS. Anal. Bioanal. Chem. 393, 1043–1054 (2009)
Code of Federal Regulations. Title 21: Food and Drugs. CFR 558.15 (2011)
Zhao, L., Stevens, J.: Determination of sulfonamide antibiotics in bovine liver using agilent bond elut QuEChERS EN kits by LC/MS/MS. Agilent Technologies (2012)
Pleasance, S., Blay, P., Quilliam, M.A., O'Hara, G.: Determination of sulfonamides by liquid chromatography, ultraviolet diode array detection, and ion-spray tandem mass spectrometry with application to cultured salmon flesh. J. Chromatogr. 558, 155–173 (1991)
Fuh, M.R., Chan, S.A.: Quantitative determination of sulfonamide in meat by liquid chromatography-electrospray-mass spectrometry. Talanta 55, 1127–1139 (2001)
Wang, Z., Hop, C.E.C.A., Kim, M.-S., Huskey, S.-E.W., Baillie, T.A., Guan, Z.: The unanticipated loss of SO2 from sulfonamides in collision-induced dissociation. Rapid Commun. Mass Spectrom. 17, 81–86 (2003)
Hu, N., Liu, P., Jiang, K., Zhou, Y., Pan, Y.: Mechanism study of SO2 elimination from sulfonamides by negative electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 22, 2715–2722 (2008)
Sun, M., Dai, W., Liu, D.Q.: Fragmentation of aromatic sulfonamides in electrospray ionization mass spectrometry: elimination of SO2 via rearrangement. J. Mass Spectrom. 43, 383–393 (2007)
Xiang, Z.: Mechanism of SO2 elimination from the aromatic sulfonamide anions: a theoretical study. Comp. Theor. Chem. 991, 74–81 (2012)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery Jr., J.A., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., Pople, J.A.: Gaussian 03, Revision C.02. Gaussian, Inc, Wallingford (2004)
Becke, A.D.: Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993)
Lee, C., Yang, W., Parr, R.G.: Development of the Colle-Salvetti correlation energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988)
Foresman, J.B., Frisch, A.E.: Exploring Chemistry with Electronic Structure Methods, 2nd edn, p. 64. Gaussian Inc, Pittsburgh (1996)
Görner, H.: Photoinduced oxygen uptake of diphenylamines in solution and their ring closure revisited. J. Phys. Chem. A 112, 1245–1250 (2008)
Encinas, S., Bosca, F., Miranda, M.A.: Photochemistry of 2,6-dichlorodiphenylamine and 1-chlorocarbazole, the photoactive chromophores of diclofenac, meclofenamic acid, and their major photoproducts. Photochem. Photobiol. 68, 640–645 (1998)
Karni, M., Mandelbaum, A.: The ‘even-electron rule’. J. Mass Spectrom. 15, 53–64 (1980)
Chai, Y., Sun, H., Pan, Y., Sun, C.: N-centered odd-electron ions formation from collision-induced dissociation of electrospray ionization generated even-electron ions: single electron transfer via ion/neutral complex in the fragmentation of protonated N,N′-dibenzylpiperazines and protonated N-benzylpiperazines. J. Am. Soc. Mass Spectrom. 22, 1526–1533 (2011)
Cai, Y., Mo, Z., Rannulu, N.S., Guan, B., Kannupal, S., Gibb, B.C., Cole, R.B.: Characterization of an exception to the ‘even-electron rule’ upon low-energy collision induced decomposition in negative ion electrospray tandem mass spectrometry. J. Mass Spectrom. 45, 235–240 (2010)
Attygalle, A.B., García-Rubio, S., Ta, J., Meinwald, J.: Collisionally-induced dissociation mass spectra of organic sulfate anions. J. Chem. Soc., Perkin Trans 2, 498–506 (2001)
Yunfeng, C., Shifeng, G., Yuanjiang, P.: A mechanistic study of formation of radical anion from fragmentation of deprotonated N,2-diphenylacetamide derivatives in electrospray ionization tandem mass spectrometry. Acta Chim. Sinica 70, 1805–1811 (2012)
Hu, N., Tu, Y.P., Jiang, K., Pan, Y.: Intramolecular charge transfer in the gas phase: fragmentation of protonated sulfonamides in mass spectrometry. J. Org. Chem. 75, 4244–4250 (2010)
Crews, P., Rodriguez, J., Jaspers, M.: Organic Structure Analysis, p. 246. Oxford University Press, New York (1998)
Hansch, C., Leo, A., Taft, R.W.: A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991)
Acknowledgments
The authors thank Jason Bialecki for the assistance provided with preparative TLC work. They are grateful to Bristol-Myers Squibb Pharmaceutical Company (New Brunswick, NJ, USA) for the donation of the Waters Quattro Ultima mass spectrometer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hibbs, J.A., Jariwala, F.B., Weisbecker, C.S. et al. Gas-Phase Fragmentations of Anions Derived from N-Phenyl Benzenesulfonamides. J. Am. Soc. Mass Spectrom. 24, 1280–1287 (2013). https://doi.org/10.1007/s13361-013-0671-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-013-0671-4