Abstract
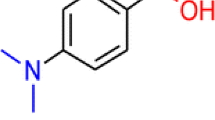
For p-(dimethylamino)chalcone (p-DMAC), the N atom is the most basic site in the liquid phase, whereas the O atom possesses the highest proton affinity in the gas phase. A novel and interesting observation is reported that the N- and O-protonated p-DMAC can be competitively produced in atmospheric pressure chemical ionization (APCI) with the change of solvents and ionization conditions. In neat methanol or acetonitrile, the protonation is always under thermodynamic control to form the O-protonated ion. When methanol/water or acetonitrile/water was used as the solvent, the protonation is kinetically controlled to form the N-protonated ion under conditions of relatively high infusion rate and high concentration of water in the mixed solvent. The regioselectivity of protonation of p-DMAC in APCI is probably attributed to the bulky solvent cluster reagent ions (SnH+) and the analyte having different preferred protonation sites in the liquid phase and gas phase.

Figure
ᅟ


Similar content being viewed by others
References
Tian, Z., Kass, S.R.: Gas-phase versus liquid-phase structures by electrospray ionization mass spectrometry. Angew. Chem. Int. Ed. 48, 1321–1323 (2009)
Schmidt, J., Meyer, M.M., Spector, I., Kass, S.R.: Infrared multiphoton dissociation spectroscopy study of protonated p-aminobenzoic acid: does electrospray ionization afford the amino- or carboxy-protonated ion? J. Phys. Chem. A 115, 7625–7632 (2011)
Tian, Z., Pawlow, A., Poutsma, J.C., Kass, S.R.: Are carboxyl groups the most acidic sites in amino acids? Gas-phase acidity, H/D exchange experiments, and computations on cysteine and its conjugate base. J. Am. Chem. Soc. 129, 5403–5407 (2007)
Tian, Z., Kass, S.R.: Does electrospray ionization produce gas-phase or liquid-phase structures? J. Am. Chem. Soc. 130, 10842–10843 (2008)
Tian, Z., Wang, X.-B., Wang, L.-S., Kass, S.R.: Are carboxyl groups the most acidic sites in amino acids? Gas-phase acidities, photoelectron spectra, and computations on tyrosine, p-hydroxybenzoic acid, and their conjugate bases. J. Am. Chem. Soc. 131, 1174–1181 (2009)
Steill, J.D., Oomens, J.: Gas-phase deprotonation of p-hydroxybenzoic acid investigated by IR spectroscopy: solution-phase structure is retained upon ESI. J. Am. Chem. Soc. 131, 13570–13571 (2009)
Schröder, D., Buděšínský, M., Roithová, J.: Deprotonation of p-hydroxybenzoic acid: does electrospray ionization sample solution or gas-phase structures? J. Am. Chem. Soc. 134, 15897–15905 (2012)
Sunner, J., Nicol, G., Kebarle, P.: Factors determining relative sensitivity of analytes in positive mode atmospheric pressure ionization mass spectrometry. Anal. Chem. 60, 1300–1307 (1988)
Andrade, F.J., Shelley, J.T., Wetzel, W.C., Webb, M.R., Gamez, G., Ray, S.J., Hieftje, G.M.: Atmospheric pressure chemical ionization source. 1. Ionization of compounds in the gas phase. Anal. Chem. 80, 2646–2653 (2008)
Terrier, P., Desmazières, B., Tortajada, J., Buchmann, W.: APCI/APPI for synthetic polymer analysis. Mass Spectrom. Rev. 30, 854–874 (2011)
Moulton, B.E., Duhme-Klair, A.K., Fairlamb, I.J.S., Lynam, J.M., Whitwood, A.C.: A rationale for the linear correlation of aryl substituent effects in iron(0) tricarbonyl complexes containing α, β-unsaturated enone (chalcone) ligands. Organometallics 26, 6354–6365 (2007)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery Jr., J.A., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., Pople, J.A.: Gaussian 03, Revision B, Gaussian, Inc., Pittsburgh, PA, 2003
Bortolini, O., Fantin, G., Ferretti, V., Fogagnolo, M., Giovannini, P.P., Medici, A.: Relative acidity scale of bile acids through ESI-MS measurements. Org. Biomol. Chem. 8, 3674–3677 (2010)
Tai, Y., Pei, S., Wan, J., Cao, X., Pan, Y.: Fragmentation study of protonated chalcones by atmospheric pressure chemical ionization and tandem mass spectrometry. Rapid Commun. Mass Spectrom. 20, 994–1000 (2006)
Hu, N., Tu, Y.-P., Liu, Y., Jiang, K., Pan, Y.: Dissociative protonation and proton transfers: fragmentation of α, β-unsaturated aromatic ketones in mass spectrometry. J. Org. Chem. 73, 3369–3376 (2008)
Hunter, E.P.L., Lias, S.G.: Evaluated gas phase basicities and proton affinities of molecules: an update. J. Phys. Chem. Ref. Data 27, 413–656 (1998)
Zhang, X.X., Oscarson, J.L., Izatt, R.M., Schuck, P.C., Li, D.: Thermodynamics of macroscopic and microscopic proton ionization from protonated 4-aminobenzoic acid in aqueous solution from 298.15 to 393.15 K. J. Phys. Chem. B 104, 8598–8605 (2000)
Olah, G.A., White, A.M.: Stable carbonium ions. LVIII. Carbon-13 resonance investigation of protonated carboxylic acids (carboxonium ions) and oxocarbonium ions (acyl cations). J. Am. Chem. Soc. 89, 7072–7075 (1967)
Laufer, D.A., Gelb, R.I., Schwartz, L.M.: 13C NMR determination of acid–base tautomerization equilibria. J. Org. Chem. 49, 691–696 (1984)
Schröder, D., Roithová, J., Gruene, P., Schwarz, H., Mayr, H., Koszinowski, K.: Unexpected gas-phase reactivity of the CH3OH adduct of Michler’s hydrol blue: proton-shuttle catalysis and stepwise radical expulsions. J. Phys. Chem. A 111, 8925–8933 (2007)
Levsen, K., Schiebel, H.-M., Terlouw, J.K., Jobst, K.J., Elend, M., Preiβ, A., Thiele, H., Ingendoh, A.: Even-electron ions: a systematic study of the neutral species lost in the dissociation of quasi-molecular ions. J. Mass Spectrom. 42, 1024–1044 (2007)
Zhang, X., Wang, H., Liao, Y., Ji, H., Guo, Y.: Study of methylation of nitrogen-containing compounds in the gas phase. J. Mass Spectrom. 42, 218–224 (2007)
Kuck, D.: Mass spectrometry of alkylbenzenes and related compounds. Part II. Gas phase ion chemistry of protonated alkylbenzenes (alkylbenzenium ions). Mass Spectrom. Rev. 9, 583–630 (1990)
Kuck, D.: Protonated aromatics and arenium ions. In: Nibbering, N.M.M. (ed.) Encyclopedia of mass spectrometry, 4, pp. 229–242. Elsevier, Amsterdam (2005)
Wood, K.V., Burinsky, D.J., Cameron, D., Cooks, R.G.: Site of gas-phase cation attachment. Protonation, methylation, and ethylation of aniline, phenol, and thiophenol. J. Org. Chem. 48, 5236–5242 (1983)
Nacson, S., Harrison, A.G., Davidson, W.R.: Effect of method of ion preparation on low-energy collision-induced dissociation mass spectra. Org. Mass Spectrom. 21, 317–319 (1986)
Mason, R., Milton, D., Harris, F.: Proton transfer to the fluorine atom in fluorobenzene: a dramatic temperature dependence in the gas phase. J. Chem. Soc. Chem. Commun. 1453–1455 (1987)
Weisz, A., Cojocaru, M., Mandelbaum, A.: Site specific gas-phase protonation of 2-t-butylmaleates and 2-t-butylsuccinates upon chemical ionization: stereochemical effects and kinetic control. J. Chem. Soc. Chem. Commun. 331–332 (1989)
Nakata, H., Suzuki, Y., Shibata, M., Takahashi, K., Konishi, H., Takeda, N., Tatematsu, A.: Chemical ionization mass spectrometry of bifunctional compounds. The behavior of bifunctional compounds on protonation. Org. Mass Spectrom. 25, 649–654 (1990)
Vais, V., Etinger, A., Mandelbaum, A.: Intramolecular proton transfers in stereoisomeric gas-phase ions and the kinetic nature of the protonation process upon chemical ionization. J. Mass Spectrom. 34, 755–760 (1999)
Denekamp, C., Mandelbaum, A.: Proton transfer via strained transition states in the elimination of alcohols from MH+ ions of stereoisomeric diethers and hydroxy esters upon chemical ionization and collision-induced dissociation. J. Mass Spectrom. 36, 422–429 (2001)
Joyce, J.R., Richards, D.S.: Kinetic Control of protonation in electrospray ionization. J. Am. Soc. Mass Spectrom. 22, 360–368 (2011)
Acknowledgments
The authors thank Dr. Y.-P. Tu, GlaxoSmithKline, for many discussions and suggestions. This work was supported by the National Science Foundation of China (no. 21025207).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic material.
ESM 1
(PDF 740 kb)
Rights and permissions
About this article
Cite this article
Chai, Y., Hu, N. & Pan, Y. Kinetic and Thermodynamic Control of Protonation in Atmospheric Pressure Chemical Ionization. J. Am. Soc. Mass Spectrom. 24, 1097–1101 (2013). https://doi.org/10.1007/s13361-013-0626-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-013-0626-9