Abstract
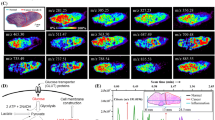
Mass spectrometric imaging (MSI) in combination with electrospray mass spectrometry (ESI-MS) is a powerful technique for visualization and identification of a variety of different biomolecules directly from thin tissue sections. As commonly used tools for molecular reporting, fluorescent proteins are molecular reporter tools that have enabled the elucidation of a multitude of biological pathways and processes. To combine these two approaches, we have performed targeted MS analysis and MALDI-MSI visualization of a tandem dimer (td)Tomato red fluorescent protein, which was expressed exclusively in the hypoxic regions of a breast tumor xenograft model. For the first time, a fluorescent protein has been visualized by both optical microscopy and MALDI-MSI. Visualization of tdTomato by MALDI-MSI directly from breast tumor tissue sections will allow us to simultaneously detect and subsequently identify novel molecules present in hypoxic regions of the tumor. MS and MALDI-MSI of fluorescent proteins, as exemplified in our study, is useful for studies in which the advantages of MS and MSI will benefit from the combination with molecular approaches that use fluorescent proteins as reporters.




Similar content being viewed by others
References
Chughtai, K., Heeren, R.M.: Mass spectrometric imaging for biomedical tissue analysis. Chem. Rev. 110, 3237–3277 (2010)
Campbell, R.E., Tour, O., Palmer, A.E., Steinbach, P.A., Baird, G.S., Zacharias, D.A., Tsien, R.Y.: A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. U. S. A. 99, 7877–7882 (2002)
Matz, M.V., Fradkov, A.F., Labas, Y.A., Savitsky, A.P., Zaraisky, A.G., Markelov, M.L., Lukyanov, S.A.: Fluorescent proteins from nonbioluminescent Anthozoa species. Nat. Biotechnol. 17, 969–973 (1999)
Gross, L.A., Baird, G.S., Hoffman, R.C., Baldridge, K.K., Tsien, R.Y.: The structure of the chromophore within DsRed, a red fluorescent protein from coral. Proc. Natl. Acad. Sci. U. S. A. 97, 11990–11995 (2000)
Baird, G.S., Zacharias, D.A., Tsien, R.Y.: Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proc. Natl. Acad. Sci. U. S. A. 97, 11984–11989 (2000)
Shaner, N.C., Campbell, R.E., Steinbach, P.A., Giepmans, B.N., Palmer, A.E., Tsien, R.Y.: Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 (2004)
Morris, L.M., Klanke, C.A., Lang, S.A., Lim, F.Y., Crombleholme, T.M.: TdTomato and EGFP identification in histological sections: insight and alternatives. Biotech. Histochem. 85, 379–387 (2010)
Winnard Jr., P.T., Kluth, J.B., Raman, V.: Noninvasive optical tracking of red fluorescent protein-expressing cancer cells in a model of metastatic breast cancer. Neoplasia 8, 796–806 (2006)
Deliolanis, N.C., Kasmieh, R., Wurdinger, T., Tannous, B.A., Shah, K., Ntziachristos, V.: Performance of the red-shifted fluorescent proteins in deep-tissue molecular imaging applications. J. Biomed. Opt. 13, 044008 (2008)
Raman, V., Artemov, D., Pathak, A.P., Winnard Jr., P.T., McNutt, S., Yudina, A., Bogdanov Jr., A., Bhujwalla, Z.M.: Characterizing vascular parameters in hypoxic regions: a combined magnetic resonance and optical imaging study of a human prostate cancer model. Cancer Res. 66, 9929–9936 (2006)
Krishnamachary, B., Penet, M.F., Nimmagadda, S., Mironchik, Y., Raman, V., Solaiyappan, M., Semenza, G.L., Pomper, M.G., Bhujwalla, Z.M.: Hypoxia regulates CD44 and its variant isoforms through HIF-1α in triple negative breast cancer. PLoS ONE 7(8): e44078 (2012)
Chughtai, K., Jiang, L., Greenwood, T.R., Klinkert, I., Amstalden van Hove, E.R., Heeren, R.M., Glunde, K.: Fiducial markers for combined 3-dimensional mass spectrometric and optical tissue imaging. Anal. Chem. 84, 1817–1823 (2012)
Jiang, L., Greenwood, T.R., Artemov, D., Raman, V., Winnard, Jr., P.T., Heeren, R.M., Bhujwalla, Z.M., Glunde, K.: Localized hypoxia results in spatially heterogeneous metabolic signatures in breast tumor models. Neoplasia 14, 732–741 (2012)
Jiang, L., Greenwood, T.R., Amstalden van Hove, E.R., Chughtai, K., Raman, V., Winnard, Jr., P.T., Heeren, R.M.A., Artemov, D., Glunde, K.: Combined magnetic resonance, fluorescence, and histology imaging strategy in a human breast tumor xenograft model. NMR Biomed. doi:10.1002/nbm.2846(2012)
Stoeckli, M., Staab, D., Schweitzer, A.: Compound and metabolite distribution measured by MALDI mass spectrometric imaging in whole-body tissue sections. Int. J. Mass Spectrom. 260, 195–202 (2007)
Surrey, T., Jahnig, F.: Refolding and oriented insertion of a membrane protein into a lipid bilayer. Proc. Natl. Acad. Sci. U. S. A. 89, 7457–7461 (1992)
Cheley, S., Malghani, M.S., Song, L., Hobaugh, M., Gouaux, J.E., Yang, J., Bayley, H.: Spontaneous oligomerization of a staphylococcal alpha-hemolysin conformationally constrained by removal of residues that form the transmembrane beta-barrel. Protein Eng. 10, 1433–1443 (1997)
Chiang, C.F., Okou, D.T., Griffin, T.B., Verret, C.R., Williams, M.N.: Green fluorescent protein rendered susceptible to proteolysis: positions for protease-sensitive insertions. Arch. Biochem. Biophys. 394, 229–235 (2001)
Alvarez, L.A., Merola, F., Erard, M., Rusconi, F.: Mass spectrometry-based structural dissection of fluorescent proteins. Biochemistry 48, 3810–3812 (2009)
Acknowledgments
This work is part of the research program of the “Foundation for Fundamental Research on Matter (FOM),” which is financially supported by the “The Netherlands Organization for Scientific Research (NWO).” The authors gratefully acknowledge financial support from NIH grant R01 CA134695. They also gratefully acknowledge continued support from The Netherlands Proteomics Centre (NPC). The authors are indebted to A.F. Maarten Altelaar and Albert J.R. Heck for enabling LC-ESI-MS/MS experiments in the NPC facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chughtai, K., Jiang, L., Post, H. et al. Mass Spectrometric Imaging of Red Fluorescent Protein in Breast Tumor Xenografts. J. Am. Soc. Mass Spectrom. 24, 711–717 (2013). https://doi.org/10.1007/s13361-012-0503-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-012-0503-y