Abstract
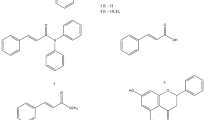
Piper ribersoides Wall. (Piperaceae), which is called “Khua Sa khan” in the local language, is mainly grown in Laos. This plant is used as a food in Laos, but no report on its metabolites exists. Crushed stems were immersed in methanol. The ethyl acetate fraction showed potential insect antifeedant activity for Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae), and some of the active compounds were piperine analogs. Piperine and its geometrical isomer independently showed potent antifeedant activities, and piperine was presumably the main active compound in the methanol extract. Interestingly, we discovered that the antifeedant activity was reduced when they were mixed (50:50).


Similar content being viewed by others
References
Escoubas P, Fukushi Y, Lajide L, Mizutani J (1992) A new method for fast isolation of insect antifeedant compounds from complex mixtures. J Chem Ecol 18:1819–1832
Friedman M, Levin CE, Lee S-U, Lee J-S, Ohnishi-Kameyama M, Kozukue N (2008) Analysis by HPLC and LC/MS of pungent piperamides in commercial black, white, green, and red whole and ground peppercorns. J Agric Food Chem 56:3028–3036
Hashimoto K, Yaoi T, Koshiba H, Yoshida T, Maoka T, Fujiwara Y, Yamamoto Y, Mori K (1996) Photochemical isomerization of piperine, a pungent constituent in pepper. Food Sci Technol Int 2:24–29
Joao A-J, Emidio D-C, Maria OC, Alexander IG (1997) Piperdardine, a piperidine alkaloid from Piper tuberculatum. Phytochemistry 44:559–561
Li Y, Zhang Y, Huang Z, Cao X, Gao K (2004) Stereoselective synthesis of naturally occurring unsaturated amide alkaloids by a modified Ramberg-Backlund reaction. Can J Chem 82:622–630
Morimoto M, Fukumoto H, Hiratani M, Chavasiri W, Komai K (2006) Insect antifeedants, pterocarpans and prerocarpol, in heartwood of Pterocarpus macrocarpus Kruz. Biosci Biotechnol Biochem 70:1864–1868
Parmar VS, Jain SC, Bisht KS, Jain R, Taneja P, Jha A, Tyagi OD, Prasad AK, Wengel J, Olsen CE, Boll PM (1997) Phytochemistry of the genus Piper. Phyrochemistry 46:597–673
Scott IM, Puniani E, Jensen H, Livesey JF, Poveda L, Sânchez-Vindas P, Durst T, Arnaso JT (2005) Analysis of Piperaceae Germplasm by HPLC and LCMS: a method for isolating and identifying unsaturated amides from Piper spp extracts. J Agric Food Chem 53:1907–1913
Singh G, Marimuthu P, Catalan C, de Lampasona MP (2004) Chemical, antioxidant and antifungal activities of volatile oil of black pepper and its acetone extract. J Sci Food Agric 84:1878–1884
Termes W, Krause EL (2002) Characterization and determination of piperine and piperine isomers in eggs. Anal Bioanal Chem 374:155–160
Wu S, Sun C, Pei S, Lu Y, Pan Y (2004) Preparative isolation and purification of amides from the fruits of Piper longum L. by upright counter-current chromatography and reversed-phase liquid chromatography. J Chromatography A 1040:193–204
Yang Y-C, Lee S-G, Lee H-K, Kim M-K, Lee S-Y, Lee H-S (2002) A piperidine amide extracted form Piper longum L. fruit shows activity against Aedes aegypti Mosquito larvae. J Agric Food Chem 50:3765–3767
Acknowledgments
The authors express sincere gratitude to Professor Emeritus Seiji Sawada, Kyoto University of Education, for supporting our research. This research was partially supported by the Ministry of Education, Science, Sports and Culture, Grant-in-Aid for Scientific Research (B), 17406003, 2005.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
1; 1H NMR (CDCl3): δ 1.62 (m, 4H), 1.65 (m, 2H), 3.58 (brm, 4H), 5.97 (s, 2H), 6.43 (d, 1H, J = 14.6 Hz), 6.73 (m, 1H), 6.74 (m, 1H), 6.78 (d, 1H, J = 8.1 Hz), 6.89 (dd, 1H, J = 1.4 and 8.1 Hz), 6.98 (d, 1H, J = 1.4 Hz), 7.31 (m, 1H).
2; 1H NMR (CDCl3): δ 1.60 (m, 4H), 1.65 (m, 2H), 3.56 (brm, 4H), 5.97 (s, 2H), 5.98 (d, 1H, J = 11.5 Hz), 6.50 (m, 1H), 6.62 (m, 1H), 6.76 (d, 1H, J = 8.1), 6.90 (dd, 1H, J = 1.7 and 8.1 Hz), 7.04 (d, 1H, J = 1.7 Hz), 7.43 (m, 1H).
3; 1H NMR (CDCl3): 1.62 (m, 4H), 1.65 (m, 2H), 2.66 (t, 2H, J = 7.5 Hz), 3.56 (brm, 4H), 6.05 (dt, 1H, J = 6.9 and 15.0 Hz), 6.19 (dd, 1H, J = 10.2 and 15.0 Hz), 6.26 (d, 1H, J = 15.0 Hz), 6.61 (dd, 1H, J = 1.8 and 7.8 Hz), 6.66 (d, 1H, J = 1.8 Hz), 6.72 (d, 1H, J = 7.8 Hz), 7.21 (dd, 1H, J = 10.2 and 15.0 Hz).
4; 1H NMR (CDCl3): δ 1.52 (m, 4H), 1.58 (m, 4H), 1.67 (m, 2H), 2.21 (m, 2H), 2.25 (m, 2H), 3.56 (brm, 4H), 5.95 (s, 2H), 6.08 (m, 1H), 6.27 (d, 1H, J = 15.0 Hz), 6.30 (d, 1H, J = 15.0 Hz), 6.75 (d, 1H, J = 8.0), 6.78 (dd, 1H, J = 1.6 and 8.0 Hz), 6.84 (m, 1H), 6.90 (d, 1H, J = 1.6 Hz).
Rights and permissions
About this article
Cite this article
Kitayama, T., Yasuda, K., Kihara, T. et al. Piperine analogs in a hydrophobic fraction from Piper ribersoides (Piperaceae) and its insect antifeedant activity. Appl Entomol Zool 48, 455–459 (2013). https://doi.org/10.1007/s13355-013-0204-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-013-0204-4