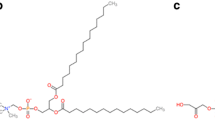
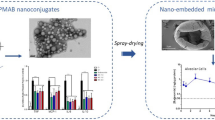
Abstract
Due to the unique physiological barriers within the lungs, there are considerable challenges in developing drug delivery systems enabling prolonged drug exposure to respiratory epithelial cells. Here, we report a PulmoSphere-based dry powder technology that incorporates a drug-phospholipid complex to promote intracellular retention of dehydroandrographolide succinate (DAS) in respiratory epithelial cells following pulmonary delivery. The DAS-phospholipid complex has the ability to self-assemble into nanoparticles. After spray-drying to produce PulmoSphere microparticles loaded with the drug-phospholipid complex, the rehydrated microparticles discharge the phospholipid complex without altering its physicochemical properties. The microparticles containing the DAS-phospholipid complex exhibit remarkable aerodynamic properties with a fine particle fraction of ∼ 60% and a mass median aerodynamic diameter of ∼ 2.3 μm. These properties facilitate deposition in the alveolar region. In vitro cell culture and lung tissue explants experiments reveal that the drug-phospholipid complex prolongs intracellular residence time and lung tissue retention due to the slow intracellular disassociation of drug from the complex. Once deposited in the lungs, the DAS-phospholipid complex loaded microparticles increase and extend drug exposure to the lung tissues and the immune cells compared to the free DAS counterpart. The improved drug exposure to airway epithelial cells, but not immune cells, is related to a prolonged duration of pulmonary anti-inflammation at decreased doses in a mouse model of acute lung injury induced by lipopolysaccharide. Overall, the phospholipid complex loaded microparticles present a promising approach for improved treatment of respiratory diseases, e.g. pneumonia and acute respiratory distress syndrome.
Graphical Abstract






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Ruaro B, Salton F, Braga L, Wade B, Confalonieri P, Volpe MC, Baratella E, Maiocchi S, Confalonieri M. The history and mystery of alveolar epithelial type II cells: focus on their physiologic and pathologic role in lung. Int J Mol Sci. 2021;22(5):2566. https://doi.org/10.3390/ijms22052566.
Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med. 2008;358(7):716–27. https://doi.org/10.1056/NEJMra074111.
Raviv AS, Alyan M, Egorov E, Zano A, Harush MY, Pieters C, Korach-Rechtman H, Saadya A, Kaneti G, Nudelman I, Farkash S, Flikshtain OD, Mekies LN, Koren L, Gal Y, Dor E, Shainsky J, Shklover J, Adir Y, Schroeder A. Lung targeted liposomes for treating ARDS. J Control Release. 2022;346:421–33. https://doi.org/10.1016/j.jconrel.2022.03.028.
Patton JS, Fishburn CS, Weers JG. The lungs as a portal of entry for systemic drug delivery. Proc Am Thorac Soc. 2004;1(4):338–44. https://doi.org/10.1513/pats.200409-049TA.
Loira-Pastoriza C, Todoroff J, Vanbever R. Delivery strategies for sustained drug release in the lungs. Adv Drug Deliv Rev. 2014;75:81–91. https://doi.org/10.1016/j.addr.2014.05.017.
Honeybourne D, Baldwin DR. The site concentrations of antimicrobial agents in the lung. J Antimicrob Chemother. 1992;30(3):249–60. https://doi.org/10.1093/jac/30.3.249.
Strong P, Ito K, Murray J, Rapeport G. Current approaches to the discovery of novel inhaled medicines. Drug Discov Today. 2018;23(10):1705–17. https://doi.org/10.1016/j.drudis.2018.05.017.
Wang Y, Chen L. Lung tissue distribution of drugs as a key factor for COVID-19 treatment. Br J Pharmacol. 2020;177(21):4995–6. https://doi.org/10.1111/bph.15102.
Calfee CS, Janz DR, Bernard GR, May AK, Kangelaris KN, Matthay MA, Ware LB. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest. 2015;147(6):1539–48. https://doi.org/10.1378/chest.14-2454.
Liang Z, Ni R, Zhou J, Mao S. Recent advances in controlled pulmonary drug delivery. Drug Discov Today. 2015;20(3):380–9. https://doi.org/10.1016/j.drudis.2014.09.020.
Hu X, Yang FF, Liao YH. Pharmacokinetic considerations of inhaled pharmaceuticals for systemic delivery. Curr Pharm Des. 2016;22(17):2532–48. https://doi.org/10.2174/1381612822666160128150005.
Gupta V, Gupta N, Shaik IH, Mehvar R, McMurtry IF, Oka M, Nozik-Grayck E, Komatsu M, Ahsan F. Liposomal fasudil, a rho-kinase inhibitor, for prolonged pulmonary preferential vasodilation in pulmonary arterial hypertension. J Control Release. 2013;167(2):189–99. https://doi.org/10.1016/j.jconrel.2013.01.011.
Anderson CF, Chakroun RW, Su H, Mitrut RE, Cui H. Interface-enrichment-induced instability and drug-loading-enhanced stability in inhalable delivery of supramolecular filaments. ACS Nano. 2019;13(11):12957–68. https://doi.org/10.1021/acsnano.9b05556.
Garbuzenko OB, Kuzmov A, Taratula O, Pine SR, Minko T. Strategy to enhance lung cancer treatment by five essential elements: inhalation delivery, nanotechnology, tumor-receptor targeting, chemo- and gene therapy. Theranostics. 2019;9(26):8362–76. https://doi.org/10.7150/thno.39816.
Kirtane AR, Verma M, Karandikar P, Furin J, Langer R, Traverso G. Nanotechnology approaches for global infectious diseases. Nat Nanotechnol. 2021;16(4):369–84. https://doi.org/10.1038/s41565-021-00866-8.
La Zara D, Sun F, Zhang F, Franek F, Balogh Sivars K, Horndahl J, Bates S, Brännström M, Ewing P, Quayle MJ, Petersson G, Folestad S, van Ommen JR. Controlled pulmonary delivery of carrier-free budesonide dry powder by atomic layer deposition. ACS Nano. 2021;15(4):6684–98. https://doi.org/10.1021/acsnano.0c10040.
Ma SQ, Cong ZQ, Wei JX, Chen WY, Ge D, Yang FF, Liao YH. Pulmonary delivery of size-transformable nanoparticles improves tumor accumulation and penetration for chemo-sonodynamic combination therapy. J Control Release. 2022;350:132–45. https://doi.org/10.1016/j.jconrel.2022.08.003.
Jones RM, Neef N. Interpretation and prediction of inhaled drug particle accumulation in the lung and its associated toxicity. Xenobiotica. 2012;42(1):86–93. https://doi.org/10.3109/00498254.2011.632827.
Healy AM, Amaro MI, Paluch KJ, Tajber L. Dry powders for oral inhalation free of lactose carrier particles. Adv Drug Deliv Rev. 2014;75:32–52. https://doi.org/10.1016/j.addr.2014.04.005.
Ong W, Nowak P, Cu Y, Schopf L, Bourassa J, Enlow E, Moskowitz SM, Chen H. Sustained pulmonary delivery of a water-soluble antibiotic without encapsulating carriers. Pharm Res. 2016;33(3):563–72. https://doi.org/10.1007/s11095-015-1808-x.
Meers P, Neville M, Malinin V, Scotto AW, Sardaryan G, Kurumunda R, Mackinson C, James G, Fisher S, Perkins WR. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic pseudomonas aeruginosa lung infections. J Antimicrob Chemother. 2008;61(4):859–68. https://doi.org/10.1093/jac/dkn059.
Okusanya OO, Bhavnani SM, Hammel J, Minic P, Dupont LJ, Forrest A, Mulder GJ, Mackinson C, Ambrose PG, Gupta R. Pharmacokinetic and pharmacodynamic evaluation of liposomal amikacin for inhalation in cystic fibrosis patients with chronic pseudomonal infection. Antimicrob Agents Chemother. 2009;53(9):3847–54. https://doi.org/10.1128/AAC.00872-08.
Cipolla D, Shekunov B, Blanchard J, Hickey A. Lipid-based carriers for pulmonary products: preclinical development and case studies in humans. Adv Drug Deliv Rev. 2014;75:53–80. https://doi.org/10.1016/j.addr.2014.05.001.
Chen WY, Wang YS, Liu CY, Ji YB, Yang FF, Liao YH. Comparison of pulmonary availability and anti-inflammatory effect of dehydroandrographolide succinate via intratracheal and intravenous administration. Eur J Pharm Sci. 2020;147:105290. https://doi.org/10.1016/j.ejps.2020.105290.
Weers J, Tarara T. The PulmoSphere™ platform for pulmonary drug delivery. Ther Deliv. 2014;5(3):277–95. https://doi.org/10.4155/tde.14.3.
Hirst PH, Pitcairn GR, Weers JG, Tarara TE, Clark AR, Dellamary LA, Hall G, Shorr J, Newman SP. In vivo lung deposition of hollow porous particles from a pressurized metered dose inhaler. Pharm Res. 2002;19(3):258–64. https://doi.org/10.1023/a:1014482615914.
Geller DE, Weers J, Heuerding S. Development of an inhaled dry-powder formulation of tobramycin using PulmoSphere™ technology. J Aerosol Med Pulm Drug Deliv. 2011;24(4):175–82. https://doi.org/10.1089/jamp.2010.0855.
Israel S, Kumar A, DeAngelis K, Aurivillius M, Dorinsky P, Roche N, Usmani OS. Pulmonary deposition of budesonide/glycopyrronium/formoterol fumarate dihydrate metered dose inhaler formulated using co-suspension delivery technology in healthy male subjects. Eur J Pharm Sci. 2020;153:105472. https://doi.org/10.1016/j.ejps.2020.105472.
Bombardelli E, Curri SB, Loggia Rd, Negro PD, Gariboldi P, Tubaro A. Complexes between phospholipids and vegetal derivates of biological interest. Fitoterapia. 1989;60:1–9.
Khan J, Alexander A, Ajazuddin, Saraf S, Saraf S. Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives. J Control Release. 2013;168(1):50–60. https://doi.org/10.1016/j.jconrel.2013.02.025.
Barani M, Sangiovanni E, Angarano M, Rajizadeh MA, Mehrabani M, Piazza S, Gangadharappa HV, Pardakhty A, Mehrbani M, Dell’Agli M, Nematollahi MH. Phytosomes as innovative delivery systems for phytochemicals: a comprehensive review of literature. Int J Nanomed. 2021;16:6983–7022. https://doi.org/10.2147/IJN.S318416.
Cook RO, Pannu RK, Kellaway IW. Novel sustained release microspheres for pulmonary drug delivery. J Control Release. 2015;104(1):79–90. https://doi.org/10.1016/j.jconrel.2005.01.003.
Wei JX, Li CY, Chen WY, Cong YJ, Liu CY, Yang FF, Liao YH. The pulmonary biopharmaceutics and anti-inflammatory effects after intratracheal and intravenous administration of re-du-ning injection. Biomed Pharmacother. 2023;160:114335. https://doi.org/10.1016/j.biopha.2023.114335.
Chen H, Kim S, He W, Wang H, Low PS, Park K, Cheng JX. Fast release of lipophilic agents from circulating PEG-PDLLA micelles revealed by in vivo forster resonance energy transfer imaging. Langmuir. 2008;24(10):5213–7. https://doi.org/10.1021/la703570m.
Chaurasiya B, Zhou M, Tu J, Sun C. Design and validation of a simple device for insufflation of dry powders in a mice model. Eur J Pharm Sci. 2018;123:495–501. https://doi.org/10.1016/j.ejps.2018.08.010.
Qiu Y, Liao Q, Chow MYT, Lam JKW. Intratracheal administration of dry powder formulation in mice. J Vis Exp. 2020;161:e61469. https://doi.org/10.3791/61469.
Rayamajhi M, Redente EF, Condon TV, Gonzalez-Juarrero M, Riches DW, Lenz LL. Non-surgical intratracheal instillation of mice with analysis of lungs and lung draining lymph nodes by flow cytometry. J Vis Exp. 2011;51:e2702. https://doi.org/10.3791/2702.
Furuie H, Saisho Y, Yoshikawa T, Shimada J. Intrapulmonary pharmacokinetics of s-013420, a novel bicyclolide antibacterial, in healthy Japanese subjects. Antimicrob Agents Chemother. 2010;54(2):866–70. https://doi.org/10.1128/AAC.00567-09.
Fu TT, Cong ZQ, Zhao Y, Chen WY, Liu CY, Zheng Y, Yang FF, Liao YH. Fluticasone propionate nanosuspensions for sustained nebulization delivery: an in vitro and in vivo evaluation. Int J Pharm. 2019;572:118839. https://doi.org/10.1016/j.ijpharm.2019.118839.
Angelico R, Ceglie A, Sacco P, Colafemmina G, Ripoli M, Mangia A. Phyto-liposomes as nanoshuttles for water-insoluble silybin-phospholipid complex. Int J Pharm. 2014;471(1–2). https://doi.org/10.1016/j.ijpharm.2014.05.026. 173– 81.
Oomens J, Steill JD. Free carboxylate stretching modes. J Phys Chem A. 2008;112(15):3281–3. https://doi.org/10.1021/jp801806e.
Lv Y, Guo Y, Luo X, Li H. Infrared spectroscopic study on chemical and phase equilibrium in triethylammonium acetate. Sci China Chem. 2012;55:1688–94. https://doi.org/10.1007/s11426-012-4634-6.
Nie H, Mo H, Zhang M, Song Y, Fang K, Taylor LS, Li T, Byrn SR. Investigating the interaction pattern and structural elements of a drug-polymer complex at the molecular level. Mol Pharm. 2015;12(7):2459–68. https://doi.org/10.1021/acs.molpharmaceut.5b00162.
Chi C, Zhang C, Liu Y, Nie H, Zhou J, Ding Y. Phytosome-nanosuspensions for silybin-phospholipid complex with increased bioavailability and hepatoprotection efficacy. Eur J Pharm Sci. 2020;144:105212. https://doi.org/10.1016/j.ejps.2020.105212.
Pomerenke A, Lea SR, Herrick S, Lindsay MA, Singh D. Characterization of tlr-induced inflammatory responses in copd and control lung tissue explants. Int J Chron Obstruct Pulmon Dis. 2016;11:2409–17. https://doi.org/10.2147/COPD.S105156.
Ayyar VS, Song D, DuBois DC, Almon RR, Jusko WJ. Modeling corticosteroid pharmacokinetics and pharmacodynamics, part I: determination and prediction of dexamethasone and methylprednisolone tissue binding in the rat. J Pharmacol Exp Ther. 2019;370(2):318–26. https://doi.org/10.1124/jpet.119.257519.
Van Holsbeke C, De Backer J, Vos W, Marshall J. Use of functional respiratory imaging to characterize the effect of inhalation profile and particle size on lung deposition of inhaled corticosteroid/long-acting β2-agonists delivered via a pressurized metered-dose inhaler. Ther Adv Respir Dis. 2018;12:1753466618760948. https://doi.org/10.1177/1753466618760948.
Jain H, Bairagi A, Srivastava S, Singh SB, Mehra NK. Recent advances in the development of microparticles for pulmonary administration. Drug Discov Today. 2020;25(10):1865–72. https://doi.org/10.1016/j.drudis.2020.07.018.
Chen T, He B, Tao J, He Y, Deng H, Wang X, Zheng Y. Application of förster resonance energy transfer (fret) technique to elucidate intracellular and in vivo biofate of nanomedicines. Adv Drug Deliv Rev. 2019;143:177–205. https://doi.org/10.1016/j.addr.2019.04.009.
Knapp S. LPS and bacterial lung inflammation models. Drug Discovery Today: Disease Models. 2009;6(4):113–8. https://doi.org/10.1016/j.ddmod.2009.08.003.
Chen H, Bai C, Wang X. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Rev Respir Med. 2010;4(6):773–83. https://doi.org/10.1586/ers.10.71.
Aeffner F, Bolon B, Davis IC. Mouse models of acute respiratory distress syndrome: a review of analytical approaches, pathologic features, and common measurements. Toxicol Pathol. 2015;43(8):1074–92. https://doi.org/10.1177/0192623315598399.
Qiao Q, Liu X, Yang T, Cui K, Kong L, Yang C, Zhang Z. Nanomedicine for acute respiratory distress syndrome: the latest application, targeting strategy, and rational design. Acta Pharm Sin B. 2021;11(10):3060–91. https://doi.org/10.1016/j.apsb.2021.04.023.
Ho DK, Nichols BLB, Edgar KJ, Murgia X, Loretz B, Lehr CM. Challenges and strategies in drug delivery systems for treatment of pulmonary infections. Eur J Pharm Biopharm. 2019;144:110–24. https://doi.org/10.1016/j.ejpb.2019.09.002.
Moldoveanu B, Otmishi P, Jani P, Walker J, Sarmiento X, Guardiola J, Saad M, Yu J. Inflammatory mechanisms in the lung. J Inflamm Res. 2009;2:1–11.
Wang Z, Li J, Cho J, Malik AB. Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils. Nat Nanotechnol. 2014;9(3):204–10. https://doi.org/10.1038/nnano.2014.17.
Liu C, Liu YH, Xi L, He Y, Liang YM, Mak J, Mao SR, Wang ZP, Zheng Y. Interactions of inhaled liposome with macrophages and neutrophils determine particle biofate and anti-inflammatory effect in acute lung inflammation. ACS Appl Mater Interfaces. 2023;15(1):479–93. https://doi.org/10.1021/acsami.2c17660.
Wang XD, Adler KB, Erjefalt J, Bai CX. Airway epithelial dysfunction in the development of acute lung injury and acute respiratory distress syndrome. Expert Rev Respir Med. 2007;1(1):149–55. https://doi.org/10.1586/17476348.1.1.149.
Weers J. Comparison of phospholipid-based particles for sustained release of ciprofloxacin following pulmonary administration to bronchiectasis patients. Pulm Ther. 2019;5(2):127–50. https://doi.org/10.1007/s41030-019-00104-6.
He Y, Liu C, Han R, Liang YM, Mak JCW, Zhu YH, Li HF, Zheng Y. Reducing systemic absorption and macrophages clearance of genistein by lipid-coated nanocrystals for pulmonary delivery. Chin Chem Lett. 2023;34(1):107484. https://doi.org/10.1016/j.cclet.2022.04.082.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82173983) and the CAMS Innovation Fund for Medical Sciences (CIFMS, Grant No. 2021-I2M-1-048).
Author information
Authors and Affiliations
Contributions
Chen WY: Investigation, Writing- Original draft & Formal analysis; Wei JX: Formal analysis, Validation; Yu CY: Investigation; Liu CY: Resources; Liao YH: Conceptualization, Writing- Review & Editing, Funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Animal procurement and experiments were subjected to approval by the Animal Ethics Committee of the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences & Peking Union Medical College, ethical approval number SLXD-20211025012.
Consent to participate
This study does not involve human subjects.
Consent for publication
This study does not contain any individual person’s data in any form.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
: More detailed information about cell viability assay, HPLC and LC-MS/MC method for DAS concentration determination. Mass spectrometry, DSC thermograms, 1H NMR spectrum of DAS-PC, UV spectra, 1H NMR spectrum and cell viability of RB-PC, and the pharmacokinetic parameters of DAS, physical mixture of DAS and SDPP, DAS@SDPP and DAS-PC@SDPP after intratracheal dosing to mice.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, WY., Wei, JX., Yu, CY. et al. Inhalable spray-dried porous microparticles containing dehydroandrographolide succinate phospholipid complex capable of improving and prolonging pulmonary anti-inflammatory efficacy in mice. Drug Deliv. and Transl. Res. (2024). https://doi.org/10.1007/s13346-024-01626-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s13346-024-01626-6