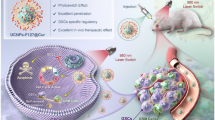
Abstract
We have previously identified a latent interaction mechanism between non-small cell lung cancer cells (NSCLCC) and their associated macrophages (TAM) mediated by mutual paracrine activation of the HMGB1/RAGE/NF-κB signaling. Activation of this mechanism results in TAM stimulation and PD-L1 upregulation in the NSCLCC. In the present work, we found that free DOX at a low concentration that does not cause DNA damage could activate the HMGB1/RAGE/NF-κB/PD-L1 pathway byinducing oxidative stress. It was thus proposed that a combination of low-dose DOX and a PD-L1 blocker delivered in the NSCLC tumor would achieve synergistic TAM stimulation and thereby synergetic anti-tumor potency. To prove this idea, DOX and BMS-202 (a PD-L1 blocker) were loaded to black phosphorus (BP) nanoparticles after dosage titration to yield the BMS-202/DOX@BP composites that rapidly disintegrated and released drug cargo upon mild photothermal heating at 40 °C. In vitro experiments then demonstrated that low-dose DOX and BMS-202 delivered via BMS-202/DOX@BP under mild photothermia displayed enhanced tumor cell toxicity with a potent synergism only in the presence of TAM. This enhanced synergism was due to an anti-tumor M1-like TAM phenotype that was synergistically induced by low dose DOX plus BMS-202 only in the presence of the tumor cells, indicating the damaged tumor cells to be the cardinal contributor to the M1-like TAM stimulation. In vivo, BMS-202/DOX@BP under mild photothermia exhibited targeted delivery to NSCLC graft tumors in mice and synergistic anti-tumor efficacy of delivered DOX and BMS-202. In conclusion, low-dose DOX in combination with a PD-L1 blocker is an effective strategy to turn TAM against their host tumor cells exploiting the HMGB1/RAGE/NF-κB/PD-L1 pathway. The synergetic actions involved highlight the value of TAM and the significance of modulating tumor cell-TAM cross-talk in tumor therapy. Photothermia-responsive BP provides an efficient platform to translate this strategy into targeted, efficacious tumor therapy.
Graphical Abstract













Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sarode P, Schaefer MB, Grimminger F, Seeger W, Savai RJFIO. Macrophage and tumor cell cross-talk is fundamental for lung tumor progression: we need to talk. Front Oncol. 2020;10:324.
Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, Rich JN. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol. 2015;17:170–82.
Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. 2014;6:1670–90.
Larionova I, Tuguzbaeva G, Ponomaryova A, Stakheyeva M, Cherdyntseva N, Pavlov V, Choinzonov E, Kzhyshkowska J. Tumor-associated macrophages in human breast, colorectal, lung, ovarian and prostate cancers. Front Oncol. 2020;10:566511.
He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660–9.
Sharma P, Allison JPJS. The future of immune checkpoint therapy. 2015;348:56–61.
Toor SM, Nair VS, Decock J, Elkord E. Immune checkpoints in the tumor microenvironment. In: Seminars in cancer biology. Elsevier; 2020. p. 1–12.
Dong Y, Sun Q, Zhang X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarger. 2017;8:2171.
Jiang Y, Chen M, Nie H, Yuan YJHV. PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Hum Vaccin Immunother. 2019;15:1111–22.
Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology. 2012;1(8):1223–5.
Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–9.
Ruan J, Ouyang M, Zhang W, Luo Y, Zhou D. Oncology, The effect of PD-1 expression on tumor-associated macrophage in T cell lymphoma. Clin Transl Oncol. 2021;23:1134–41.
Kono Y, Saito H, Miyauchi W, Shimizu S, Murakami Y, Shishido Y, Miyatani K, Matsunaga T, Fukumoto Y, Nakayama Y. Increased PD-1-positive macrophages in the tissue of gastric cancer are closely associated with poor prognosis in gastric cancer patients. BMC Cancer. 2020;20:1–9.
Dhupkar P, Gordon N, Stewart J, Kleinerman ES. Anti-PD-1 therapy redirects macrophages from an M2 to an M1 phenotype inducing regression of OS lung metastases. Cancer Med. 2018;7:2654–64.
Rao G, Latha K, Ott M, Sabbagh A, Marisetty A, Ling X, Zamler D, Doucette TA, Yang Y, Kong LY. Anti–PD-1 induces M1 polarization in the glioma microenvironment and exerts therapeutic efficacy in the absence of CD8 cytotoxic T cells. Clin Cancer Res. 2020;26:4699–712.
Lu D, Ni Z, Liu X, Feng S, Dong X, Shi X, Zhai J, Mai S, Jiang J, Wang Z. Beyond T cells: understanding the role of PD-1/PD-L1 in tumor-associated macrophages. J Immunol Res. 2019;2019.
Liu Y, Zugazagoitia J, Ahmed FS, Henick BS, Gettinger SN, Herbst RS, Schalper KA, Rimm DL. Immune cell PD-L1 colocalizes with macrophages and is associated with outcome in PD-1 pathway blockade therapy. Clin Cancer Res. 2020;26:970–7.
Chen L, Cao MF, Xiao JF, Ma QH, Zhang H, Cai RL, Miao JY, Wang WY, Zhang H, Luo M, Ping YF. Stromal PD-1+ tumor-associated macrophages predict poor prognosis in lung adenocarcinoma. Hum Pathol. 2020;97:68–79.
Zhang W, Liu Y, Yan Z, Yang H, Sun W, Yao Y, Chen Y, Jiang R. IL-6 promotes PD-L1 expression in monocytes and macrophages by decreasing protein tyrosine phosphatase receptor type O expression in human hepatocellular carcinoma. JITC. 2020;8(1).
Fang W, Zhou T, Shi H, Yao M, Zhang D, Qian H, Zeng Q, Wang Y, Jin F, Chai C, Chen T. Progranulin induces immune escape in breast cancer via up-regulating PD-L1 expression on tumor-associated macrophages (TAMs) and promoting CD8+ T cell exclusion. J Exp Clin Cancer Res. 2021;40:1–11.
Loeuillard E, Yang J, Buckarma E, Wang J, Liu Y, Conboy C, Pavelko KD, Li Y, O’Brien D, Wang C. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. JCI. 2020;130:5380–96.
McCord R, Bolen CR, Koeppen H, Kadel EE III, Oestergaard MZ, Nielsen T, Sehn LH, Venstrom JM. PD-L1 and tumor-associated macrophages in de novo DLBCL. Blood Adv. 2019;3:531–40.
Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. PNAS. 2017;114:1117–22.
Hartley GP, Chow L, Ammons DT, Wheat WH, Dow SW. Programmed cell death ligand 1 (PD-L1) signaling regulates macrophage proliferation and activation. Cancer Immunol Res. 2018;6:1260–73.
Zhu Z, Zhang H, Chen B, Liu X, Zhang S, Zong Z, Gao M. PD-L1-mediated immunosuppression in glioblastoma is associated with the infiltration and M2-polarization of tumor-associated macrophages. Front Immunol. 2020;11:588552.
Shinchi Y, Ishizuka S, Komohara Y, Matsubara E, Mito R, Pan C, Yoshii D, Yonemitsu K, Fujiwara Y, Ikeda K. The expression of PD-1 ligand 1 on macrophages and its clinical impacts and mechanisms in lung adenocarcinoma. CII. 2022;71(2022):2645–61.
Chen S, Crabill GA, Pritchard TS, McMiller TL, Wei P, Pardoll DM, Pan F, Topalian SL. Mechanisms regulating PD-L1 expression on tumor and immune cells. JITC. 2019;7:1–12.
Vegliante MC, Mazzara S, Zaccaria GM, De Summa S, Esposito F, Melle F, Motta G, Sapienza MR, Opinto G, Volpe G. NR1H3 (LXRα) is associated with pro-inflammatory macrophages, predicts survival and suggests potential therapeutic rationales in diffuse large b-cell lymphoma. Hematol Oncol. 2022;40:864–75.
Zhao R, Wan Q, Wang Y, Wu Y, Xiao S, Li Q, Shen X, Zhuang W, Zhou Y, Xia L. M1-like TAMs are required for the efficacy of PD-L1/PD-1 blockades in gastric cancer. Oncoimmunology. 2021;10:1862520.
Sun N-Y, Chen Y-L, Wu W-Y, Lin H-W, Chiang Y-C, Chang C-F, Tai Y-J, Hsu H-C, Chen CA, Sun WZ. Blockade of PD-L1 enhances cancer immunotherapy by regulating dendritic cell maturation and macrophage polarization. Cancer. 2019;11:1400.
Xu H-Z, Li T-F, Wang C, Ma Y, Liu Y, Zheng M-Y, Liu Z-J-Y, Chen J-B, Li K, Sun SK. Synergy of nanodiamond–doxorubicin conjugates and PD-L1 blockade effectively turns tumor-associated macrophages against tumor cells. J Nanobiotechnology. 2021;19:1–24.
Rawat PS, Jaiswal A, Khurana A, Bhatti JS, Navik U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed Pharmacother. 2021;139:111708.
Damodar G, Smitha T, Gopinath S, Vijayakumar S, Rao YA. An evaluation of hepatotoxicity in breast cancer patients receiving injection Doxorubicin. Ann Med Res. 2014;4:74–9.
Barakat BM, Ahmed HI, Bahr HI, Elbahaie M. Protective effect of boswellic acids against doxorubicin-induced hepatotoxicity: impact on Nrf2/HO-1 defense pathway. Oxidative medicine and cellular longevity. 2018;2018.
Su C, Wang H, Liu Y, Guo Q, Zhang L, Li J, Zhou W, Yan Y, Zhou X, Zhang J. Adverse effects of anti-PD-1/PD-L1 therapy in non-small cell lung cancer. Front Oncol. 2020;10:1821.
Hu Y-B, Zhang Q, Li H-J, Michot JM, Liu H-B, Zhan P, Lv T-F, Song Y. Evaluation of rare but severe immune related adverse effects in PD-1 and PD-L1 inhibitors in non-small cell lung cancer: a meta-analysis. TLCR. 2017;6:S8.
Zhou X, Yao Z, Bai H, Duan J, Wang Z, Wang X, Zhang X, Xu J, Fei K, Zhang Z. Treatment-related adverse events of PD-1 and PD-L1 inhibitor-based combination therapies in clinical trials: a systematic review and meta-analysis. Lancet Oncol. 2021;22:1265–74.
Songbo M, Lang H, Xinyong C, Bin X, Ping Z, Liang S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol Lett. 2019;307:41–8.
Sangomla S, Saifi MA, Khurana A, Godugu C. Biology nanoceria ameliorates doxorubicin induced cardiotoxicity: Possible mitigation via reduction of oxidative stress and inflammation. JTEMIN. 2018;47:53–62.
Kong C-Y, Guo Z, Song P, Zhang X, Yuan Y-P, Teng T, Yan L, Tang Q-Z. Underlying the mechanisms of doxorubicin-induced acute cardiotoxicity: oxidative stress and cell death. Int J Biol Sci. 2022;18:760.
Jiang Y, Zhang J, Qiu J, Cui S. The PD-1/PD-L1 binding inhibitor BMS-202 suppresses the synthesis and secretion of gonadotropins and enhances apoptosis via p38 MAPK signaling pathway. Drug Dev Res. 2022;83:176–83.
Yang X, Wang W, Ji T. Metabolic Remodeling by the PD-L1 Inhibitor BMS-202 Significantly inhibits cell malignancy in human glioblastomas. Cell Death Dis. 2024;15(3):186.
Padmanabhan R, Kheraldine H, Gupta I, Meskin N, Hamad A, Vranic S, Al Moustafa AE. Quantification of the growth suppression of HER2+ breast cancer colonies under the effect of trastuzumab and PD-1/PD-L1 inhibitor. Front Oncol. 2022;12:977664.
Padmanabhan R, Kheraldine H, Gupta I, Meskin N, Hamad A, Vranic S, Al Moustafa AE. Mathematical modeling of the growth suppression of HER2+ breast cancer colonies under the effect of trastuzumab and PD-1/PD-L1 inhibitor, (2022).
Anaya-Ruiz M, Perez-Santos M. Small-molecule inhibitor PD-1/PD-L1 interaction for colorectal cancer treatment. Pharm Pat Anal. 2021;10:245–50.
Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63:131–5.
Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, Moreira PI. Doxorubicin: the good, the bad and the ugly effect. Curr Med. 2009;16:3267–85.
Johnson-Arbor K, Dubey R. Doxorubicin, (2017).
Srivastava A, Amreddy N, Babu A, Panneerselvam J, Mehta M, Muralidharan R, Chen A, Zhao YD, Razaq M, Riedinger N. Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells. Sci Rep. 2016;6:38541.
Doroshow JH. Role of hydrogen peroxide and hydroxyl radical formation in the killing of Ehrlich tumor cells by anticancer quinones. PNAS. 1986;83:4514–8.
Escors D, Gato-Cañas M, Zuazo M, Arasanz H, García-Granda MJ, Vera R, Kochan G. The intracellular signalosome of PD-L1 in cancer cells. Signal transduction and targeted therapy. 2018;3:26.
Gato-Cañas M, Zuazo M, Arasanz H, Ibañez-Vea M, Lorenzo L, Fernandez-Hinojal G, Vera R, Smerdou C, Martisova E, Arozarena I. PDL1 signals through conserved sequence motifs to overcome interferon-mediated cytotoxicity. Cell Rep. 2017;20:1818–29.
Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561.
Hamanishi J, Mandai M, Matsumura N, Abiko K, Baba T, Konishi I. PD-1/PD-L1 blockade in cancer treatment: perspectives and issues. Int J Clin Oncol. 2016;21:462–73.
Hao Q, Idell S, Tang H. M1 macrophages are more susceptible to necroptosis. J Cell Immunol. 2021;3:97.
Ren D, Hua Y, Yu B, Ye X, He Z, Li C, Wang J, Mo Y, Wei X, Chen Y. Predictive biomarkers and mechanisms underlying resistance to PD1/PD-L1 blockade cancer immunotherapy. Mol Cancer. 2020;19:1–19.
Ashizawa T, Iizuka A, Tanaka E, Kondou R, Miyata H, Maeda C, Sugino T, Yamaguchi K, Ando T, Ishikawa Y. Antitumor activity of the PD-1/PD-L1 binding inhibitor BMS-202 in the humanized MHC-double knockout NOG mouse. Biomed Res. 2019;40:243–50.
Shang Q, Zhou S, Jiang Y, Wang D, Wang J, Song A, Luan Y. Rational design of a robust antibody-like small-molecule inhibitor nanoplatform for enhanced photoimmunotherapy. ACS Appl Mater Interfaces. 2020;12:40085–93.
Tu K, Yu Y, Wang Y, Yang T, Hu Q, Qin X, Tu J, Yang C, Kong L, Zhang Z. Combination of chidamide-mediated epigenetic modulation with immunotherapy: Boosting tumor immunogenicity and response to PD-1/PD-L1 blockade. ACS Appl Mater Interfaces. 2021;13:39003–17.
Wang Y, Yu J, Li D, Zhao L, Sun B, Wang J, Wang Z, Zhou S, Wang M, Yang Y. Paclitaxel derivative-based liposomal nanoplatform for potentiated chemo-immunotherapy. JCR. 2022;341:812–27.
Yao Y, Chen H, Tan N. Cancer-cell-biomimetic nanoparticles systemically eliminate hypoxia tumors by synergistic chemotherapy and checkpoint blockade immunotherapy. APSB. 2022;12:2103–19.
Kuntz KL, Wells RA, Hu J, Yang T, Dong B, Guo H, Woomer AH, Druffel DL, Alabanza A, Tománek D. Control of surface and edge oxidation on phosphorene. ACS Appl Mater Interfaces. 2017;9:9126–35.
Tao W, Zhu X, Yu X, Zeng X, Xiao Q, Zhang X, Ji X, Wang X, Shi J, Zhang H, Mei L. Black phosphorus nanosheets as a robust delivery platform for cancer theranostics. 2017;29:1603276.
Qiu M, Wang D, Liang W, Liu L, Zhang Y, Chen X, Sang DK, Xing C, Li Z, Dong BJ. Novel concept of the smart NIR-light–controlled drug release of black phosphorus nanostructure for cancer therapy. PNAS. 2018;115:501–6.
Ling Z, Li P, Zhang SY, Arif N, Zeng YJ. Stability and passivation of 2D group VA elemental materials: black phosphorus and beyond. J Condens Matter Phys. 2022;34:224004.
Qiu S, Liang J, Hou Y, Zhou X, Zhou Y, Wang J, Zou B, Xing W, Hu Y. Hindered phenolic antioxidant passivation of black phosphorus affords air stability and free radical quenching. J Colloid Interface Sci. 2022;606:1395–409.
Li T-F, Li K, Wang C, Liu X, Wen Y, Xu Y-H, Zhang Q, Zhao Q-Y, Shao M, Li Y-ZJJOCR. Harnessing the cross-talk between tumor cells and tumor-associated macrophages with a nano-drug for modulation of glioblastoma immune microenvironment. JCR. 2017;268:128–46.
Toda G, Yamauchi T, Kadowaki T, Ueki KJSP. Preparation and culture of bone marrow-derived macrophages from mice for functional analysis. STAR Protoc. 2021;2:100246.
Olive PL, Banáth JPJNP. The comet assay: a method to measure DNA damage in individual cells. Nat Protoc. 2006;1:23–9.
Funding
This research was supported by the National Natural Science Foundation of China [82272718].
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design. H-ZX and F-XC conducted experiments in vitro and in vivo. KL, QZ, NH made data analysis. T-FL and Y-HX conducted part of the experiments. YC and XC designed and supervised the work. The first draft of the manuscript was written by XC, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The animal study was reviewed and approved by the Animal Care Committee at Wuhan University.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
All authors give consent for publication.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, HZ., Chen, FX., Li, K. et al. Anti-lung cancer synergy of low-dose doxorubicin and PD-L1 blocker co-delivered via mild photothermia-responsive black phosphorus. Drug Deliv. and Transl. Res. (2024). https://doi.org/10.1007/s13346-024-01595-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s13346-024-01595-w