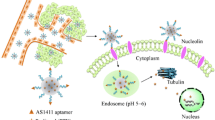
Abstract
Targeted nanodelivery systems offer a promising approach to cancer treatment, including the most common cancer in women, breast cancer. In this study, a targeted, pH-responsive, and biocompatible nanodelivery system based on nucleolin aptamer-functionalized biogenic titanium dioxide nanoparticles (TNP) was developed for targeted co-delivery of FOXM1 aptamer and doxorubicin (DOX) to improve breast cancer therapy. The developed targeted nanodelivery system exhibited almost spherical morphology with 124.89 ± 12.97 nm in diameter and zeta potential value of − 23.78 ± 3.66 mV. FOXM1 aptamer and DOX were loaded into the nanodelivery system with an efficiency of 100% and 97%, respectively. Moreover, the targeted nanodelivery system demonstrated excellent stability in serum and a pH-responsive sustained drug release profile over a period of 240 h following Higuchi kinetic and Fickian diffusion mechanism. The in vitro cytotoxicity experiments demonstrated that the targeted nanodelivery system provided selective internalization and strong growth inhibition effects of about 45 and 51% against nucleolin-positive 4T1 and MCF-7 breast cancer cell lines. It is noteworthy that these phenomena were not observed in nucleolin-negative cells (CHO). The preclinical studies revealed that a single-dose intravenous injection of the targeted nanodelivery system into 4T1-bearing mice inhibited tumor growth by 1.7- and 1.4-fold more efficiently than the free drug and the non-targeted nanodelivery system, respectively. Our results suggested that the developed innovative targeted pH-responsive biocompatible nanodelivery system could serve as a prospectively potential platform to improve breast cancer treatment.
Graphical Abstract








Similar content being viewed by others
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding authors on reasonable request.
References
Rani R, Malik P, Dhania S, Mukherjee TK. Recent advances in mesoporous silica nanoparticle-mediated drug delivery for breast cancer treatment. Pharmaceutics. 2023;15:227.
Ye D, Wang Y, Deng X, Zhou X, Liu D, Zhou B, Zheng W, Wang X, Fang L. DNMT3a-dermatopontin axis suppresses breast cancer malignancy via inactivating YAP. Cell Death Dis. 2023;14:106.
Chen L, Zhan M, Li J, Cao L, Sun H, Laurent R, Mignani S, Caminade A-M, Majoral J-P, Shi X. Amphiphilic phosphorous dendron micelles co-deliver microRNA inhibitor and doxorubicin for augmented triple negative breast cancer therapy. J Mater Chem B. 2023;11:5483–93.
Espinoza MJC, Lin KS, Weng MT, Kunene SC, Lin Y-S, Liu S-Y. Magnetic boron nitride nanosheets-based on ph-responsive smart nanocarriers for the delivery of doxorubicin for liver cancer treatment. Colloids Surf B. 2023;222:113129.
Sawpari R, Samanta S, Banerjee J, Das S, Dash SS, Ahmed R, Giri B, Dash SK. Recent advances and futuristic potentials of nano-tailored doxorubicin for prostate cancer therapy. J Drug Deliv Sci Technol. 2023;81:104212.
Shahriari M, Taghdisi SM, Abnous K, Ramezani M, Alibolandi M. Self-targeted polymersomal co-formulation of doxorubicin, camptothecin and FOXM1 aptamer for efficient treatment of non-small cell lung cancer. J Control Release. 2021;335:369–88.
Fathi-Karkan S, Mirinejad S, Ulucan-Karnak F, Mukhtar M, Almanghadim HG, Sargazi S, Rahdar A, Díez-Pascual AM. Biomedical applications of aptamer-modified chitosan nanomaterials: an updated review. Int J Biol Macromol. 2023;238:124103.
Sheikh A, Md S, Alhakamy NA, Kesharwani P. Recent development of aptamer conjugated chitosan nanoparticles as cancer therapeutics. Int J Pharm. 2022;620:121751.
Fang RH, Gao W, Zhang L. Targeting drugs to tumours using cell membrane-coated nanoparticles, Nature Reviews. Clin Oncol. 2023;20:33–48.
Patra JK, Das G, Fraceto LF, Campos EV, Rodriguez-Torres MD, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16:71.
Albalawi F, Hussein MZ, Fakurazi S, Masarudin MJ. Engineered nanomaterials: the challenges and opportunities for nanomedicines. Int J Nanomedicine. 2021:161–184.
Hasannia M, Lamei K, Abnous K, Taghdisi SM, Nekooei S, Nekooei N, Ramezani M, Alibolandi M. Targeted poly (L-glutamic acid)-based hybrid peptosomes co-loaded with doxorubicin and USPIONs as a theranostic platform for metastatic breast cancer, Nanomedicine: Nanotechnology. Biol Med 2023;48:102645.
Chandrakala V, Aruna V, Angajala G. Review on metal nanoparticles as nanocarriers: current challenges and perspectives in drug delivery systems. Emergent Materials. 2022;5:1593–615.
Lim XY, Capinpin SM, Bolem N, Foo AS, Yip WC, Kumar AP, Teh DB. Biomimetic nanotherapeutics for targeted drug delivery to glioblastoma multiforme. Bioeng transl med. 2023:e10483.
Puri S, Mazza M, Roy G, England RM, Zhou L, Nourian S, Subramony JA. Evolution of nanomedicine formulations for targeted delivery and controlled release. Adv Drug Deliv Rev. 2023:114962.
Rabiee N, Ahmadi S, Iravani S, Varma RS. Natural resources for sustainable synthesis of nanomaterials with anticancer applications: a move toward green nanomedicine. Environ Res. 2023;216:114803.
Alarif WM, Shaban YA, Orif MI, Ghandourah MA, Turki AJ, Alorfi HS, Tadros HR. Green synthesis of TiO2 nanoparticles using natural marine extracts for antifouling activity. Mar Drugs. 2023;21:62.
Anupong W, On-Uma R, Jutamas K, Salmen SH, Alharbi SA, Joshi D, Jhanani G. Antibacterial, antifungal, antidiabetic, and antioxidant activities potential of Coleus aromaticus synthesized titanium dioxide nanoparticles. Environ Res. 2023;216: 114714.
Mansoor A, Khurshid Z, Khan MT, Mansoor E, Butt FA, Jamal A, Palma PJ. Medical and dental applications of titania nanoparticles: an overview. Nanomaterials. 2022;12:3670.
Zhan L, Yin X, Zhang Y, Ju J, Wu Y, Ding L, Li C, Chen X, Wang Y. Polydopamine-guarded metal-organic frameworks as co-delivery systems for starvation-assisted chemo-photothermal therapy. Biomaterials Advances. 2023;146: 213306.
Zhan H, Jagtiani T, Liang JF. A new targeted delivery approach by functionalizing drug nanocrystals through polydopamine coating. Eur J Pharm Biopharm. 2017;114:221–9.
Siciliano G, Monteduro AG, Turco A, Primiceri E, Rizzato S, Depalo N, Curri ML, Maruccio G. Polydopamine-coated magnetic iron oxide nanoparticles: from design to applications. Nanomaterials. 2022;12:1145.
Lu W, Liu W, Hu A, Shen J, Yi H, Cheng Z. Combinatorial polydopamine-liposome nanoformulation as an effective anti-breast cancer therapy. Int J Nanomedicine. 2023:861–879.
Yuan X, Zhu Y, Li S, Wu Y, Wang Z, Gao R, Luo S, Shen J, Wu J, Ge L. Titanium nanosheet as robust and biosafe drug carrier for combined photochemo cancer therapy. J Nanobiotechnology. 2022;20:154.
Subhan MA, Yalamarty SSK, Filipczak N, Parveen F, Torchilin VP. Recent advances in tumor targeting via EPR effect for cancer treatment. J Pers Med. 2021;11:571.
Sindhwani S, Syed AM, Ngai J, Kingston BR, Maiorino L, Rothschild J, MacMillan P, Zhang Y, Rajesh NU, Hoang T. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19:566–75.
Zhao X, Yang J, Wang X, Chen L, Zhang C, Shen Z. Inhibitory effect of aptamer-carbon dot nanomaterial-siRNA complex on the metastasis of hepatocellular carcinoma cells by interfering with FMRP. Eur J Pharm Biopharm. 2022;174:47–55.
Taghdisi SM, Danesh NM, Nameghi MA, Bahreyni A, Ramezani M, Alibolandi M, Emrani AS, Abnous K. Co-delivery of doxorubicin and α-PCNA aptamer using AS1411-modified pH-responsive nanoparticles for cancer synergistic therapy. J Drug Deliv Sci Technol. 2020;58:101816.
Babaei M, Abnous K, Taghdisi SM, Taghavi S, Saljooghi ASh, Ramezani M, Alibolandi M. Targeted rod-shaped mesoporous silica nanoparticles for the co-delivery of camptothecin and survivin shRNA in to colon adenocarcinoma in vitro and in vivo. Eur J Pharm Biopharm. 2020;156:84–96.
Lopes-Nunes J, Lifante J, Shen Y, Ximendes EC, Jaque D, Iglesias-de la Cruz MC, Cruz C. Biological studies of an ICG-tagged aptamer as drug delivery system for malignant melanoma. Eur J Pharm Biopharm. 2020;154:228–35.
Eramabadi P, Masoudi M, Makhdoumi A, Mashreghi M. Microbial cell lysate supernatant (CLS) alteration impact on platinum nanoparticles fabrication, characterization, antioxidant and antibacterial activity. Mater Sci Eng C. 2020;117:111292.
Rezaei N, Arki MK, Miri-Lavasani Z, Solhi R, Khoramipour M, Rashedi H, Aghdaei HA, Hossein-Khannazer N, Mostafavi E, Vosough M. Co-delivery of doxorubicin and paclitaxel via noisome nanocarriers attenuates cancerous phenotypes in gastric cancer cells. Eur J Pharm Biopharm. 2023;188:33–47.
Qamar OA, Jamil F, Hussain M, Bae S, Inayat A, Shah NS, Waris A, Akhter P, Kwon EE, Park Y-K. Advances in synthesis of TiO2 nanoparticles and their application to biodiesel production: a review. Chem Eng J. 2023;460:141734.
Ramalingam V, Sundaramahalingam S, Rajaram R. Size-dependent antimycobacterial activity of titanium oxide nanoparticles against Mycobacterium tuberculosis. J Mater Chem B. 2019;7:4338–46.
Munir S, Shah SM, Hussain H. Effect of carrier concentration on the optical band gap of TiO2 nanoparticles. Mater Des. 2016;92:64–72.
Mbenga Y, Adeyemi JO, Mthiyane DM, Singh M, Onwudiwe DC. Onwudiwe, Green synthesis, antioxidant and anticancer activities of TiO2 nanoparticles using aqueous extract of Tulbhagia violacea. Results Chem. 2023:101007.
Cuadra JG, Molina-Prados S, Mínguez-Vega G, Estrada AC, Trindade T, Oliveira C, Seabra MP, Labrincha J, Porcar S, Cadena R, Fraga D, Carda JB. Multifunctional silver-coated transparent TiO2 thin films for photocatalytic and antimicrobial applications. Appl Surf Sci. 2023;617:156519.
Masoudi M, Mashreghi M, Goharshadi E, Meshkini A. Multifunctional fluorescent titania nanoparticles: green preparation and applications as antibacterial and cancer theranostic agents. Artificial Cells, Nanomedicine, and Biotechnology. 2018;46:248–59.
Barani M, Masoudi M, Mashreghi M, Makhdoumi A, Eshghi H. Cell-free extract assisted synthesis of ZnO nanoparticles using aquatic bacterial strains: biological activities and toxicological evaluation. Int J Pharm. 2021;606:120878.
Ahmadi F, Akbari J, Saeedi M, Seyedabadi M, Ebrahimnejad P, Ghasemi S, Nokhodchi A. Efficient synergistic combination effect of curcumin with piperine by polymeric magnetic nanoparticles for breast cancer treatment. J Drug Deliv Sci Technol. 2023;86:104624.
Komaraiah D, Radha E, Kalarikkal N, Sivakumar J, Reddy MR, Sayanna R. Structural, optical and photoluminescence studies of sol-gel synthesized pure and iron doped TiO2 photocatalysts. Ceram Int. 2019;45:25060–8.
Dessai S, Ayyanar M, Amalraj S, Khanal P, Vijayakumar S, Gurav N, Rarokar N, Kalaskar M, Nadaf S, Gurav S. Bioflavonoid mediated synthesis of TiO2 nanoparticles: characterization and their biomedical applications. Mater Lett. 2022;311:131639.
Fadeel DAA, Hanafy MS, Kelany NA, Elywa MA. Novel greenly synthesized titanium dioxide nanoparticles compared to liposomes in drug delivery: in vivo investigation on ehrlich solid tumor model. Heliyon. 2021;7:e07370.
Chen N, Yao S, Li M, Wang Q, Sun X, Feng X, Chen Y. Nonporous versus mesoporous bioinspired polydopamine nanoparticles for skin drug delivery. Biomacromol. 2023;24:1648–61.
Barani M, Khatami M, Behnam B, Rajendram R, Kesharwani P, Sahebkar A. Aptamer-conjugated carbon nanotubes or graphene for targeted cancer therapy and diagnosis, in: aptamers engineered nanocarriers for cancer therapy. Elsevier. 2023. pp. 277–294.
Moradi E, Zavvar T, Alibolandi M, Ramezani M, Abnous K, Taghdisi SM. Targeted delivery of epirubicin to breast cancer cells using poly-aptamer DNA nanocarriers prepared by the RCA method with multiple repeats of aptamers of FOXM1 and AS1411. Drug Dev Ind Pharm. 2023;49:260–70.
He S, Du Y, Tao H, Duan H. Advances in aptamer-mediated targeted delivery system for cancer treatment. Int J Biol Macromol. 2023;238:124173.
Dos Reis SB, de Oliveira Silva J, Garcia-Fossa F, Leite EA, Malachias A, Pound-Lana G, Mosqueira VC, Oliveira MC, de Barros AL, de Jesus MB. Mechanistic insights into the intracellular release of doxorubicin from pH-sensitive liposomes. Biomed Pharmacother. 2021;134:110952.
Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2012;65:157–70.
Jiang G, Wei C, Chen Y, Lyu Y, Huang J, Chen H, Gao X. Targeted drug delivery system inspired by macropinocytosis. J Control Release. 2023;359:302–14.
Van den Avont A, Sharma-Walia N. Anti-nucleolin aptamer AS1411: an advancing therapeutic. Front Mol Biosci. 2023;10.
Hazeri Y, Samie A, Ramezani M, Alibolandi M, Yaghoobi E, Dehghani S, Zolfaghari R, Khatami F, Zavvar T, Nameghi MA, Abnous K, Taghdisi SM. Dual-targeted delivery of doxorubicin by mesoporous silica nanoparticle coated with AS1411 aptamer and RGDK-R peptide to breast cancer in vitro and in vivo. J Drug Deliv Sci Technol. 2022;71:103285.
Hosseini NF, Amini R, Ramezani M, Saidijam M, Hashemi SM, Najafi R. AS1411 aptamer-functionalized exosomes in the targeted delivery of doxorubicin in fighting colorectal cancer. Biomed Pharmacother. 2022;155:113690.
Ghasemzadeh T, Hasannia M, Abnous K, Taghdisi SM, Nekooei S, Nekooei N, Ramezani M, Alibolandi M. Preparation of targeted theranostic red blood cell membranes-based nanobubbles for treatment of colon adenocarcinoma. Expert Opin Drug Deliv. 2023;20:131–43.
Lu Y, Chen L, Wu Z, Zhou P, Dai J, Li J, Wen Q, Fan Y, Zeng F, Chen Y, Fu S. Self-driven bioactive hybrids co-deliver doxorubicin and indocyanine green nanoparticles for chemo/photothermal therapy of breast cancer. Biomed Pharmacother. 2023;169:115846.
Hasannia M, Abnous K, Taghdisi SM, Nekooei S, Ramezani M, Alibolandi M. Synthesis of doxorubicin-loaded peptosomes hybridized with gold nanorod for targeted drug delivery and CT imaging of metastatic breast cancer. J Nanobiotechnology. 2022;20:391.
Falsafi M, Hassanzadeh Goji N, Sh. Saljooghi A, Abnous K, Taghdisi SM, Nekooei S, Ramezani M, Alibolandi M. Synthesis of a targeted, dual pH and redox-responsive nanoscale coordination polymer theranostic against metastatic breast cancer in vitro and in vivo. Expert Opin Drug Deliv. 2022;19:743–754.
Funding
This work was supported by the Ferdowsi University of Mashhad (grant number: 56126).
Ferdowsi University of Mashhad,56126,Mina Masoudi
Author information
Authors and Affiliations
Contributions
MM: conceptualization, visualization, validation, investigation, writing-original draft, and writing—review and editing; SMT: conceptualization, validation, and writing—review and editing; GH: supervision and writing—review and editing; and KA: supervision and writing—review and editing.
Corresponding authors
Ethics declarations
Ethics approval
All approved experimental protocols by the Animal Ethics Committee of Ferdowsi University of Mashhad were followed during animal experiments.
Consent for publication
All authors agreed with the journal policy and provided our consent for the publication.
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Masoudi, M., Taghdisi, S.M., Hashemitabar, G. et al. Targeted co-delivery of FOXM1 aptamer and DOX by nucleolin aptamer-functionalized pH-responsive biocompatible nanodelivery system to enhance therapeutic efficacy against breast cancer: in vitro and in vivo. Drug Deliv. and Transl. Res. 14, 1535–1550 (2024). https://doi.org/10.1007/s13346-023-01495-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-023-01495-5