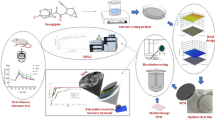
Abstract
The focus of this investigation was to determine the mechanism of effective glass transition temperature (TgE) on the crystallization behavior and microstructure of drugs in crystalline solid dispersion (CSD). CSDs were prepared by rotary evaporation using ketoconazole (KET) as a model drug and the triblock copolymer poloxamer 188 as a carrier. The pharmaceutical properties of CSDs, such as crystallite size, crystallization kinetics, and dissolution behavior, were investigated to provide a foundation for studying the crystallization behavior and the microstructure of drugs in CSDs. According to classical nucleation theory, the relationship of treatment temperature-drug crystallite size-TgE of CSD was investigated. Voriconazole, a compound that is structurally similar to KET but with different physicochemical properties, was used to verify the conclusions. The dissolution behavior of KET was significantly enhanced compared to the raw drug due to smaller crystallite size. Crystallization kinetic studies revealed a two-step crystallization mechanism for KET-P188-CSD, in which P188 crystallized first and KET crystallized later. When the treatment temperature was near TgE, the drug crystallite size was smaller and more numerous, which suggests nucleation and slow growth. With the increase of temperature, the drug changed from nucleation to growth, and the number of crystallites decreased and the size of the drug increased. This result suggests it is possible to prepare CSDs with higher drug loading and smaller crystallite size by adjusting the treatment temperature and TgE, so as to maximize the drug dissolution rate. The VOR-P188-CSD maintained a relationship between treatment temperature, drug crystallite size, and TgE. The findings of our study demonstrate that TgE and the treatment temperature can be used to regulate the drug crystallite size and improve the drug solubility and dissolution rate.
Graphical Abstract










Similar content being viewed by others
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Coty J-B, Martin C, Telò I, Spitzer D. Use of spray flash evaporation (SFE) technology to improve dissolution of poorly soluble drugs: case study on furosemide nanocrystals. Int J Pharm. 2020;589:119827.
Xun Z, Long L, Xiang G, Hui Z, Jing G, Yimeng D, Xiaowu H, Aiping Z. In vitro evaluation of quercetin nanocrystals with different particle sizes. J Nanosci Nanotechnol. 2020;20(10):6469–74.
Edueng K, Kabedev A, Ekdahl A, Mahlin D, Baumann J, Mudie D, Bergström CA. Pharmaceutical profiling and molecular dynamics simulations reveal crystallization effects in amorphous formulations. Int J Pharm. 2021;613:121360.
Junqiu Z, Qingguo L, Huahua X, Tiantian S, Yu-E W, Wenhai H, Yan M, Shixia G. An aseptic one-shot bottom-up method to produce progesterone nanocrystals: controlled size and improved bioavailability. Mol Pharm. 2019;16(12):5076–84.
Shawn XY, Miriam F, Jinling C, Alice H, Sandy J, Tu L, Munir H, Ronald S. Bioavailability enhancement of a COX-2 inhibitor, BMS-347070, from a nanocrystalline dispersion prepared by spray-drying. J Pharm Sci. 2005;94(7):1598–607.
Qian F, Jing T, Desikan S, Hussain M, Smith RL. Mechanistic investigation of Pluronic based nano-crystalline drug-polymer solid dispersions. Pharm Res. 2007;24(8):1551–60.
Hu C, Liu Z, Liu C, Zhang Y, Fan H, Qian F. Improvement of antialveolar echinococcosis efficacy of albendazole by a novel nanocrystalline formulation with enhanced oral bioavailability. ACS Infect Dis. 2020;6(5):802–10.
Zhu Q, Taylor LS, Harris MT. Evaluation of the microstructure of semicrystalline solid dispersions. Mol Pharm. 2010;7(4):1291–300.
Baird JA, Taylor LS. Evaluation and modeling of the eutectic composition of various drug-polyethylene glycol solid dispersions. Pharm Dev Technol. 2011;16(3):201–11.
Qing Z, Harris MT, Taylor LS. Modification of crystallization behavior in drug/polyethylene glycol solid dispersions. Mol Pharm. 2012;9(3):546–53.
Qing Z, Toth SJ, Simpson GJ, Hsu H-Y, Taylor LS, Harris MT. Correction to “Crystallization and dissolution behavior of naproxen/polyethylene glycol solid dispersions.” J Phys Chem B. 2013;117(17):5393.
Chen Z, Liu C, Zhang L, Qian F. Dissolution characteristics of fast-crystallizing β-lapachone within different semi-crystalline microstructures of PEG or PEO-PPO-PEO triblock copolymeR. Cryst Growth Des. 2016;16(9):5367–76.
Nassiri-Kashani M, Namdar R, Nafisi S, Maibach HI. Improved voriconazole topical delivery by nanoparticles (minireview). Pharm Chem J. 2016;50(2):76–9.
Che J, Wu Z, Shao W, Guo P, Lin Y, Pan W, Zeng W, Zhang G, Wu C, Xu Y. Synergetic skin targeting effect of hydroxypropyl-β-cyclodextrin combined with microemulsion for ketoconazole. Eur J Pharm Biopharm. 2015;93:136–48.
Aca B, Ag A, Kh A, Tm A. Exploring novel carrier for improving bioavailability of Itraconazole; solid dispersion through Hot Melt extrusion. J Drug Deliv Sci Technol. 2021;63:102541.
Rambla-Alegre M, Esteve-Romero J, Carda-Broch S. Is it really necessary to validate an analytical method or not? That is the question. J Chromatogr A. 2012;1232(7):101–9.
Guangjiao Y, Tao F, Guoqin Z, Meiling C, Fan L, Lili S, Meng W, Xiaoliang R. Preparation, optimization, characterization and in vitro release of baicalein-solubilizing glycyrrhizic acid nano-micelles. Int J Pharm. 2021;601:120546.
Bhatt V, Shete G, Bansal AK. Mechanism of generation of drug nanocrystals in celecoxib: mannitol nanocrystalline solid dispersion. Int J Pharm. 2015;495(1):132–9.
Chen Y, Wang S, Wang S, Liu C, Su C, Hageman M, Hussain M, Haskell R, Stefanski K, Qian F. Initial drug dissolution from amorphous solid dispersions controlled by polymer dissolution and drug-polymer interaction. Pharm Res. 2016;33(10):2445–58.
Siepmann J, Siepmann F. Mathematical modeling of drug dissolution. Int J Pharm. 2013;453(1):12–24.
Pardhi V, Verma T, Flora S, Chandasana H, Shukla R. Nanocrystals: an overview of fabrication, characterization and therapeutic applications in drug delivery. Curr Pharm Des. 2019;24(43):5129–46.
Jamoussi Y, Zaiter T, Desrumaux C, Acar N, Béduneau A. Investigation of the spontaneous nanoemulsification process with medium- and long-chain triglycerides. Colloids Surf B Biointerfaces. 2021;197:111432–41.
De WH, Hessels Martin JT, Boon M, Sjollema KA, Hinrichs Wouter LJ, Eissens Anko C, Frijlink HW. CLSM as quantitative method to determine the size of drug crystals in a solid dispersion. Pharm Res. 2011;28(10):2567–74.
Ai F, Wang J, Li Y, Ma Y. Effect of drug particle size on complexation, physicochemical properties and dissolution of cyclodextrin inclusion complexes. Indian J Pharm Sci. 2017;79(1):131–8.
Jing T, Ye S, Zhang G, Lian Y. Solubility of small-molecule crystals in polymers: D-mannitol in PVP, indomethacin in PVP/VA, and nifedipine in PVP/VA. Pharm Res. 2009;26(4):855–64.
Qiao N, Wang K, Schlindwein W, Davies A, Li M. In situ monitoring of carbamazepine-nicotinamide cocrystal intrinsic dissolution behaviour. Eur J Pharm Biopharm. 2013;83(3):415–26.
Koparkar AD, Augsburger LL, Shangraw RF. Intrinsic dissolution rates of tablet filler-binders and their influence on the dissolution of drugs from tablet formulations. Pharm Res. 1990;7(1):80–6.
Yu LX, Carlin AS, Amidon GL, Hussain AS. Feasibility studies of utilizing disk intrinsic dissolution rate to classify drugs. Int J Pharm. 2003;270(1):221–7.
Oxtoby DW. Nucleation of first-order phase transitions. Acc Chem Res. 1998;31(2):91–7.
Funding
This research was supported by the National Natural Science Foundation of China (Project No. 82060644), the 2022 Chinese Academy of Sciences “Western Light” Talent Training Program, and the Qinghai Provincial Department of Science and Technology (Project No. 2022-QY-201), along with support from Qinghai University School of Medicine.
Author information
Authors and Affiliations
Contributions
Conceptualization, experimental work, writing (draft), and writing revision, YZ; experimental work and writing (draft), LY; experimental work, QL.Y; conceptualization and manuscript revision, YL and CH.H.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. No animal studies have been performed in this research.
Consent for publication
Not applicable. No human studies have been performed in this research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Yan, Q., Liu, Y. et al. Study on the regulation mechanism of effective glass transition temperature on the crystallization of crystalline solid dispersion. Drug Deliv. and Transl. Res. 13, 2677–2689 (2023). https://doi.org/10.1007/s13346-023-01348-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-023-01348-1