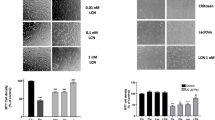
Abstract
In spinal cord injuries, axonal regeneration decreases with the activation of astrocytes followed by glial scar formation. Targeting reactive astrocytes has been recently performed by unsafe viral vectors to inhibit gliosis. In the current study, biocompatible polymeric nanoparticles were selected as an alternative for viruses to target reactive astrocytes for further drug/gene delivery applications. Lipopolysaccharide-bonded chitosan-quantum dots/poly acrylic acid nanoparticles were prepared by ionic gelation method to target reactive astrocytes both in vitro and in spinal cord-injured rats. Owing to their biocompatibility and pH-responsive behavior, chitosan and poly acrylic acid were the main components of nanoparticles. Nanoparticles were then chemically labeled with quantum dots to track the cell uptake and electrostatically interacted with lipopolysaccharide as a targeting ligand. In vitro and in vivo studies were performed in triplicate and all data were expressed as the mean ± the standard error of the mean. Smart nanoparticles with optimum size (61.9 nm) and surface charge (+ 12.5 mV) successfully targeted primary reactive astrocytes extracted from the rat cerebral cortex. In vitro studies represented high cell viability (96%) in the exposure of biocompatible nanoparticles. The pH-responsive behavior of nanoparticles was proved by their internalization into the cell’s nuclei due to the swelling and endosomal escape of nanoparticles in acidic pH. In vivo studies demonstrated higher transfection of nanoparticles into reactive astrocytes compared to the neurons. pH-responsive ligand-bonded chitosan-based nanoparticles are good alternatives for viral vectors in targeted delivery applications for the treatment of spinal cord injuries.
Graphical Abstract









Similar content being viewed by others
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–47.
Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–27.
Ben Haim L, Carrillo-de Sauvage MA, Ceyzériat K, Escartin C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci. 2015;9:278.
Vismara I, Papa S, Veneruso V, Mauri E, Mariani A, De Paola M, Affatato R, Rossetti A, Sponchioni M, Moscatelli D, Sacchetti A, Rossi F, Forloni G, Veglianese P. Selective modulation of A1 astrocytes by drug-loaded nano-structured gel in spinal cord injury. ACS Nano. 2019;14:360–71.
Su Z, Niu W, Liu ML, Zou Y, Zhang CL. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun. 2014;5:1–15.
Huang Y, Tan S. Direct lineage conversion of astrocytes to induced neural stem cells or neurons. Neurosci Bull. 2015;31:357–67.
Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy 425 of SCID-X1. J Clin Investig. 2008;118:3132–42.
Riviere CD, Douar AM. Long-term expression and repeated administration of AAV type 1, 2, and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 2006;13:1300–8.
Chou LY, Ming K, Chan WC. Strategies for the intracellular delivery of nanoparticles. Chem Soc Rev. 2011;40:233–45.
Rauch J, Kolch W, Laurent S, Mahmoudi M. Big signals from small particles: regulation of cell signaling pathways by nanoparticles. Chem Rev. 2013;113:3391–406.
Salatin S, Maleki Dizaj S, Yari KA. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biol Int. 2015;39:881–90.
Cheng R, Meng F, Deng C, Klok HA, Zhong Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials. 2013;34:3647–57.
Kurakhmaeva KB, Djindjikhashvili IA, Petrov VE, Balabanyan VU, Voronina TA, Trofimov SS, Kreuter J, Gelperina S, Begley D, Alyautdin RN. Brain targeting of nerve growth factor using poly (butyl cyanoacrylate) nanoparticles. J Drug Target. 2009;17:564–74.
Huang M, Zhang SF, Lü S, Qi T, Yan J, Gao C, Liu M, Li T, Ji Y. Synthesis of mesoporous silica/polyglutamic acid peptide dendrimer with dual targeting and its application in dissolving thrombus. J Biomed Mater Res A. 2019;107:1824–31.
Ren H, Han M, Zhou J, Zheng ZF, Lu P, Wang JJ, Wang JQ, Mao QJ, Gao JQ, Ouyang HW. Repair of spinal cord injury by inhibition of astrocyte growth and inflammatory factor synthesis through local delivery of flavopiridol in PLGA nanoparticles. Biomaterials. 2014;35:6585–94.
Ouyang Q, Meng Y, Zhou W, Tong J, Cheng Z, Zhu Q. New advances in brain-targeting nano-drug delivery systems for Alzheimer’s disease. J Drug Target. 2022;30:61–81.
Tığlı Aydın RS, Kaynak G, Gümüşderelioğlu M. Salinomycin encapsulated nanoparticles as a targeting vehicle for glioblastoma cells. J Biomed Mater Res A. 2016;104:455–64.
Furman JL, Sama DM, Gant JC, Beckett TL, Murphy MP, Bachstetter AD, Van Eldik LJ, Norris CM. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J Neurosci. 2012;32:16129–40.
Colin A, Faideau M, Dufour N, Auregan G, Hassig R, Andrieu T, Brouillet E, Hantraye P, Bonvento G, Déglon N. Engineered lentiviral vector targeting astrocytes in vivo. Glia. 2009;57:667–79.
Cao S, Jiang Y, Levy CN, Hughes SM, Zhang H, Hladik F, Woodrow KA. Optimization and comparison of CD4-targeting lipid–polymer hybrid nanoparticles using different binding ligands. J Biomed Mater Res A. 2018;106:1177–88.
Li X, Kozielski K, Cheng YH, Liu H, Zamboni CG, Green J, Mao HQ. Nanoparticle-mediated conversion of primary human astrocytes into neurons and oligodendrocytes. Biomater Sci. 2016;4:1100–12.
Gu J, Al-Bayati K, Ho EA. Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytes. Drug Deliv Transl Res. 2017;7:497–506.
Nance E, Porambo M, Zhang F, Mishra MK, Buelow M, Getzenberg R, Johnston M, Kannan RM, Fatemi A, Kannan S. Systemic dendrimer-drug treatment of ischemia-induced neonatal white matter injury. J Control Release. 2015;214:112–20.
Proulx J, Joshi C, Vijayaraghavalu S, Saraswathy M, Labhasetwar V, Ghorpade A, Borgmann K. Arginine-modified polymers facilitate poly (lactide-co-glycolide)-based nanoparticle gene delivery to primary human astrocytes. Int J Nanomed. 2020;15:3639.
Francis R, Kumar DS. Biomedical applications of polymeric materials and composites. John Wiley & Sons. 2016;1–12.
Patel T, Zhou J, Piepmeier JM, Saltzman WM. Polymeric nanoparticles for drug delivery to the central nervous system. Adv Drug Deliv Rev. 2012;64:701–5.
You JO, Almeda D, Ye GJ, Auguste DT. Bioresponsive matrices in drug delivery. J Biol Eng. 2010;4:1–13.
Wu D, Delair T. Stabilization of chitosan/hyaluronan colloidal polyelectrolyte complexes in physiological conditions. Carbohydr Polym. 2015;119:149–58.
Kiang T, Bright C, Cheung CY, Stayton PS, Hoffman AS, Leong KW. Formulation of chitosan-DNA nanoparticles with poly (propyl acrylic acid) enhances gene expression. J Biomater Sci Polym Ed. 2004;15:1405–21.
Hu Y, Ding Y, Ding D, Sun M, Zhang L, Jiang X, Yang C. Hollow chitosan/poly (acrylic acid) nanospheres as drug carriers. Biomacromol. 2007;8:1069–76.
Ping Y, Liu CD, Tang GP, Li JS, Li J, Yang WT, Xu FJ. Functionalization of chitosan via atom transfer radical polymerization for gene delivery. Adv Funct Mater. 2010;20:3106–16.
Sato T, Nakata M, Yang Z, Torizuka Y, Kishimoto S, Ishihara M. In vitro and in vivo gene delivery using chitosan/hyaluronic acid nanoparticles: influences of molecular mass of hyaluronic acid and lyophilization on transfection efficiency. J Gene Med. 2017;19:2968.
Lallana E, Rios de la Rosa JM, Tirella A, Pelliccia M, Gennari A, Stratford IJ, Puri S, Ashford M, Tirelli N. Chitosan/hyaluronic acid nanoparticles: rational design revisited for RNA delivery. Mol Pharm. 2017;14:2422–36.
Luther EM, Koehler Y, Diendorf J, Epple M, Dringen R. Accumulation of silver nanoparticles by cultured primary brain astrocytes. Nanotechnology. 2011;22:375101.
Mansur HS, Mansur AA, Curti E, De Almeida MV. Functionalized-chitosan/quantum dot nano-hybrids for nanomedicine applications: towards bio labeling and absorbing phosphate metabolites. J Mater Chem B. 2013;1:1696–711.
Saucier-Sawyer JK, Deng Y, Seo YE, Cheng CJ, Zhang J, Quijano E, Saltzman WM. Systemic delivery of blood-brain barrier-targeted polymeric nanoparticles enhances delivery to brain tissue. J Drug Target. 2015;23:736–49.
Pascual-Lucas M, Fernandez-Lizarbe S, Montesinos J, Guerri C. LPS or ethanol triggers clathrin-and rafts/caveolae-dependent endocytosis of TLR4 in cortical astrocytes. J Neurochem. 2014;129:448–62.
Santos-Carballal B, Fernández Fernández E, Goycoolea F. Chitosan in non-viral gene delivery: role of structure, characterization methods, and insights in cancer and rare diseases therapies. Polymers. 2018;10:444.
Mansur AA, Mansur HS, Soriano-Araujo A, Lobato ZI. Fluorescent nanohybrids based on quantum dot–chitosan–antibody as potential cancer biomarkers. ACS Appl Mater Interfaces. 2014;6:11403–12.
Imran M, Ehrhardt CJ, Bertino MF, Shah MR, Yadavalli VK. Chitosan stabilized silver nanoparticles for the electrochemical detection of lipopolysaccharide: a facile biosensing approach for gram-negative bacteria. Micromachines. 2020;11:413.
Reis CP, Neufeld RJ, Ribeiro AJ, Veiga F. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine. 2006;2:8–21.
Zarei-Kheirabadi M, Mirsadeghi S, Vaccaro AR, Rahimi-Movaghar V, Kiani S. Protocol for purification and culture of astrocytes: useful not only in 2 days postnatal but also in adult rat brain. Mol Biol Rep. 2020;47:1783–94.
Yu AC, Lee Y, Eng L. Astrogliosis in culture: I. The model and the effect of antisense oligonucleotides on glial fibrillary acidic protein synthesis. J Neurosci Res. 1993;34:295–303.
Zarei-Kheirabadi M, Hesaraki M, Shojaei A, Kiani S, Baharvand H. Generation of neural stem cells from adult astrocytes by using a single reprogramming factor. J Cell Physiol. 2019;234:18697–706.
Zarei-Kheirabadi M, Hesaraki M, Kiani S, Baharvand H. In vivo conversion of rat astrocytes into neuronal cells through neural stem cells in the injured spinal cord with a single zinc-finger transcription factor. Stem Cell Res Ther. 2019;10:1–19.
Ye Q, Mettu S, Zhou M, Dagastine R, Ashokkumar M. Ultrasonically synthesized organic liquid-filled chitosan microcapsules: part 1: tuning physical & functional properties. Soft Matter. 2018;14:3202–8.
Dewald I, Isakin O, Schubert J, Kraus T, Chanana M. Protein identity and environmental parameters determine the final physicochemical properties of protein-coated metal nanoparticles. J Phys Chem C. 2015;119:25482–92.
Mili B, Das K, Kumar A, Saxena AC, Singh P, Ghosh S, Bag S. Preparation of NGF encapsulated chitosan nanoparticles and its evaluation on neuronal differentiation potentiality of canine mesenchymal stem cells. J Mater Sci Mater Med. 2018;29:1–13.
Guo L, Liu G, Hong RY, Li HZ. Preparation and characterization of chitosan poly (acrylic acid) magnetic microspheres. Mar Drugs. 2010;8:2212–22.
Kim S, Burgula Y, Ojanen-Reuhs T, Cousin MA, Reuhs BL, Mauer LJ. Differentiation of crude lipopolysaccharides from Escherichia coli strains using Fourier transform infrared spectroscopy and chemometrics. J Food Sci. 2006;71:57–61.
Kim S, Reuhs B, Mauer L. Use of Fourier transform infrared spectra of crude bacterial lipopolysaccharides and chemometrics for differentiation of Salmonella enterica serotypes. J Appl Microbiol. 2005;99:411–7.
Bezerra Neto JR, de Lima NP, Sales FA, da Silva EE, Ladeira LO, Freire VN, Caetano EW. Phosphate group vibrational signatures of the osteoporosis drug alendronate. J Raman Spectrosc. 2014;45:801–6.
Lin Y, Zhang L, Yao W, Qian H, Ding D, Wu W, Jiang X. Water-soluble chitosan-quantum dot hybrid nanospheres toward bioimaging and bio labeling. ACS Appl Mater Interfaces. 2011;3:995–1002.
Tan WB, Jiang S, Zhang Y. Quantum-dot based nanoparticles for targeted silencing of HER2/neu gene via RNA interference. Biomaterials. 2007;28:1565–71.
Tan WB, Huang N, Zhang Y. Ultrafine biocompatible chitosan nanoparticles encapsulating multi-colored quantum dots for bioapplications. J Colloid Interface Sci. 2007;310:464–70.
Shao XR, Wei XQ, Song X, Hao LY, Cai XX, Zhang ZR, Peng Q, Lin YF. Independent effect of polymeric nanoparticle zeta potential/surface charge, on their cytotoxicity and affinity to cells. Cell Prolif. 2015;48:465–74.
Nandhakumar S, Dhanaraju MD, Sundar VD, Heera B. Influence of surface charge on the in vitro protein adsorption and cell cytotoxicity of paclitaxel loaded poly (ε-caprolactone) nanoparticles. Bull Fac Pharm Cairo Univ. 2017;55:249–58.
Grabowska-Jadach I, Drozd M, Kulpińska D, Komendacka K, Pietrzak M. Modification of fluorescent nanocrystals with 6-thioguanine: monitoring of drug delivery. Appl Nanosci. 2020;10:83–93.
Yu L, Tan M, Ho B, Ding JL, Wohland T. Determination of critical micelle concentrations and aggregation numbers by fluorescence correlation spectroscopy: aggregation of a lipopolysaccharide. Anal Chim Acta. 2006;556:216–25.
Coccini T, Caloni F, Ramirez Cando LJ, De Simone U. Cytotoxicity and proliferative capacity impairment induced on human brain cell cultures after short- and long-term exposure to magnetite nanoparticles. J Appl Toxicol. 2017;37:361–73.
Au C, Mutkus L, Dobson A, Riffle J, Lalli J, Aschner M. Effects of nanoparticles on the adhesion and cell viability on astrocytes. Biol Trace Elem Res. 2007;120:248–56.
Tenenbaum M, Azab AN, Kaplanski J. Effects of estrogen against LPS-induced inflammation and toxicity in primary rat glial and neuronal cultures. J Endotoxin Res. 2007;13:158–66.
Teleanu DM, Chircov C, Grumezescu AM, Teleanu RI. Neurotoxicity of nanomaterials: an up-to-date overview. Nanomaterials. 2019;9:96.
Voigt N, Henrich-Noack P, Kockentiedt S, Hintz W, Tomas J, Sabel BA. Toxicity of polymeric nanoparticles in vivo and in vitro. J Nanoparticle Res. 2014;16:1–13.
Acknowledgements
Here, we acknowledge the Iran National Science Foundation (INSF) for supporting us in this research.
Funding
This work was supported by the Iran National Science Foundation (INSF) under Grant number [96004422]. Prof. Masoud Frounchi has received this research support from INSF.
Author information
Authors and Affiliations
Contributions
Parinaz Sabourian: substantial contribution to the conception, design of the work, the acquisition, analysis, and interpretation of data; has drafted the work and substantively revised it. Masoud Frounchi: substantial contribution to the conception and design of the work and has substantively revised the work. Sahar Kiani: substantial contribution to the conception and design of the work and has substantively revised the work. Shohreh Mashayekhan: substantial contribution to the conception and has substantively revised the work. Masoumeh Zarei Kheirabadi: analysis, interpretation of data; and has drafted the work. Yasaman Heydari: analysis, interpretation of data; and has drafted the work. Seyed Sajad Ashraf: analysis, and interpretation of data.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The animal studies were performed in line with the principles of the Declaration of the ROYAN Institute of Iran. Approval was granted by the ethics committee of the ROYAN Institute of Iran. No human studies have been performed in this research.
Consent for publication
No human studies have been performed in this research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sabourian, P., Frounchi, M., Kiani, S. et al. Targeting reactive astrocytes by pH-responsive ligand-bonded polymeric nanoparticles in spinal cord injury. Drug Deliv. and Transl. Res. 13, 1842–1855 (2023). https://doi.org/10.1007/s13346-023-01300-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-023-01300-3