Abstract
The global burden of neurological disorders has been increasing day by day which calls for immediate attention to the solutions. Novel drug delivery systems are one of the alternatives that we count on to counteract these disorders. As the blood–brain barrier creates a significant hindrance to the delivery of drugs across the endothelium lining of the brain, nose-to-brain delivery has been the favorite option to administer such drugs. In recent times, bioconjugation has been viewed as a rapidly growing area in the field of pharmaceuticals. The pharmaceutical industry and academic research are investing significantly in bioconjugated structures as an attractive and advantageous potential aid to nanoparticulate delivery systems, with all of its flexible benefits in terms of tailor grafting and custom design as well as overcoming the majority of their drawbacks. This review discusses drug delivery via the intranasal route and gives insight into bioconjugation systems for drug molecules, their chemistry, and benefits over other systems. Conjugation of drugs/macromolecules with peptides, carbohydrates, ligands, and nucleic acids has also been discussed in detail.
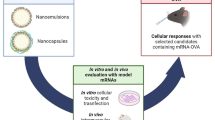
Graphical abstract
The figure represents few types of novel drug delivery systems and molecules that have been attempted by researchers for nose-to-brain delivery through nasal (mucosal) route for the effective management of epilepsy, Alzheimer’s disease, brain cancer, and other brain disorders.








Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- ADC:
-
Antibody-drug conjugate
- BBB:
-
Blood-brain barrier
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- DA:
-
Dopamine
- DIC:
-
N,N′-Diisopropylcarbodiimide
- DRG:
-
Delonix Regia Gum
- DZ:
-
Donepezil
- EDC:
-
N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide
- FBS:
-
Fetal bovine serum
- FGFR:
-
Fibroblast growth factor receptor
- HA:
-
Hyaluronic acid
- HOAt:
-
1-Hydroxy-7-azabenzotriazole
- HOBt:
-
1-Hydroxybenzotriazole
- HOPy:
-
1-Hydroxy-2-pyridinone
- HRP:
-
Horseradish peroxidase
- LUMO:
-
Lowest unoccupied molecular orbital
- MMP:
-
Matrix metalloproteinase
- NHS:
-
N-Hydroxysuccinimide
- NPs:
-
Nanoparticles
- OECs:
-
Olfactory ensheathing cells
- PAMAM:
-
Polyamidoamine
- PD:
-
Parkinson’s disease
- PDZ:
-
PAMAM 4.0 G dendrimer
- PEG:
-
Polyethylene glycol
- PLA:
-
Polylactic acid
- PLGA:
-
Polylactic-co-glycolic acid
- PM:
-
Polymeric micelles
- PTX:
-
Paclitaxel
- SNF:
-
Simulated nasal fluid
- TMP:
-
Tetramethylpyrazine
- WGA:
-
Wheat germ agglutinin
References
Elzahhar P, Belal ASF, Elamrawy F, Helal NA, Nounou MI. Bioconjugation in drug delivery: practical perspectives and future perceptions. Pharm Nanotechnol. 2019. https://doi.org/10.1007/978-1-4939-9516-5_11.
Algar WR. A brief introduction to traditional bioconjugate chemistry. Chemoselective Bioorthogonal Ligation React. 2017. https://doi.org/10.1002/9783527683451.ch1.
Kozlovskaya L, Abou-Kaoud M, Stepensky D. Quantitative analysis of drug delivery to the brain via nasal route. J Control Release. 2014;189:133–40. https://doi.org/10.1016/j.jconrel.2014.06.053.
Ul Islam S, Shehzad A, Bilal Ahmed M, Lee YS. Intranasal delivery of nanoformulations: a potential way of treatment for neurological disorders. Molecules. 2020;25(8):1–27. https://doi.org/10.3390/molecules25081929.
Djupesland PG, Messina JC, Mahmoud RA. The nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overview. Ther Deliv. 2014;5(6):709–33. https://doi.org/10.4155/tde.14.41.
Faghihnejad A, Feldman KE, Yu J, Tirrell MV, Israelachvili JN, Hawker CJ, Kramer EJ, Zeng H. Adhesion and surface interactions of a self-healing polymer with multiple hydrogen-bonding groups. Adv Funct Mater. 2014;24(16):2322–33. https://doi.org/10.1002/adfm.201303013.
Cha TW, Quo A, Zhu XY. Enzymatic activity on a chip: the critical role of protein orientation. Proteomics. 2005;5(2):416–9. https://doi.org/10.1002/pmic.200400948.
Peluso P, Wilson DS, Do D, Tran H, Venkatasubbaiah M, Quincy D, Heidecker B, Poindexter K, Tolani N, Phelan M, Witte K, Jung LS, Wagner P, Nock S. Optimizing antibody immobilization strategies for the construction of protein microarrays. Anal Biochem. 2003;312(2):113–24. https://doi.org/10.1016/S0003-2697(02)00442-6.
Liu X, Jang CH, Zheng F, Jürgensen A, Denlinger JD, Dickson KA, Raines RT, Abbott NL, Himpsel FJ. Characterization of protein immobilization at silver surfaces by near edge X-ray absorption fine structure spectroscopy. Langmuir. 2006;22(18):7719–25. https://doi.org/10.1021/la060988w.
Werengowska-Ciećwierz K, Wis̈niewski M, Terzyk AP, Furmaniak S. The Chemistry of bioconjugation in nanoparticles-based drug delivery system. Adv Condens Matter Phys. Hindawi Publishing Corporation; 2015. https://doi.org/10.1155/2015/198175.
Chalker JM, Bernardes GJL, Lin YA, Davis BG. Chemical modification of proteins at cysteine: opportunities in chemistry and biology. Chem - An Asian J. 2009;4(5):630–40. https://doi.org/10.1002/asia.200800427.
Frayne SH, Murthy RR, Northrop BH. Investigation and demonstration of catalyst/initiator-driven selectivity in thiol-Michael reactions. J Org Chem. 2017;82(15):7946–56. https://doi.org/10.1021/acs.joc.7b01200.
Kalia J, Raines R. Advances in bioconjugation. Curr Org Chem. 2009;14(2):138–47. https://doi.org/10.2174/138527210790069839.
Sheyi R, de la Torre BG, Albericio F. Linkers: an assurance for controlled delivery of antibody-drug conjugate. Pharmaceutics. 2022. https://doi.org/10.3390/pharmaceutics14020396.
Erlanson DA, Wells JA, Braisted AC. Tethering: fragment-based drug discovery. Annu Rev Biophys Biomol Struct. 2004;33:199–223. https://doi.org/10.1146/annurev.biophys.33.110502.140409.
Zou L, Liu X, Li J, Li W, Zhang L, Fu C, Zhang J, Gu Z. Redox-sensitive carrier-free nanoparticles self-assembled by disulfide-linked paclitaxel-tetramethylpyrazine conjugate for combination cancer chemotherapy. Theranostics. 2021;11(9):4171–86. https://doi.org/10.7150/thno.42260.
Zhao Y, Zhang L, Yao P, Yue Q, Hai L, Guo L, Wang Q, Wu Y. GLUT1 -mediated venlafaxine- TDS-glucose conjugates with “lock in” function for CNS delivery. Chem Biol Drug Des. 2018;91(3):707–16. https://doi.org/10.1111/cbdd.13128.
Rousselle C, Clair P, Smirnova M, Kolesnikov Y, Pasternak GW, Gac-Breton S, Rees AR, Scherrmann JM, Temsamani J. Improved brain uptake and pharmacological activity of dalargin using a peptide-vector-mediated strategy. J Pharmacol Exp Ther. 2003;306(1):371–6. https://doi.org/10.1124/jpet.102.048520.
Nilsson BL, Soellner MB, Raines RT. Chemical synthesis of proteins. Annu Rev Biophys Biomol Struct. 2005;34:91–118. https://doi.org/10.1146/annurev.biophys.34.040204.144700.
Chu BCF, Wahl GM, Orgel LE. Derivatization of unprotected polynucleotides. Nucleic Acids Res. 1983;11(18):6513–29.
El-Faham A, Albericio F. Peptide coupling reagents, more than a letter soup. Chem Rev. 2011;111(11):6557–602. https://doi.org/10.1021/cr100048w.
Valeur E, Bradley M. Amide bond formation: beyond the myth of coupling reagents. Chem Soc Rev. 2009;38(2):606–31. https://doi.org/10.1039/b701677h.
Nobs L, Buchegger F, Gurny R, Allémann E. Current methods for attaching targeting ligands to liposomes and nanoparticles. J Pharm Sci. 2004;93(8):1980–92. https://doi.org/10.1002/jps.20098.
Kocbek P, Obermajer N, Cegnar M, Kos J, Kristl J. Targeting cancer cells using PLGA nanoparticles surface modified with monoclonal antibody. J Control Release. 2007;120(1–2):18–26. https://doi.org/10.1016/j.jconrel.2007.03.012.
Mansur AAP, Carvalho SM, Lobato ZIP, Leite MDF, Cunha ADS, Mansur HS. Design and development of polysaccharide-doxorubicin-peptide bioconjugates for dual synergistic effects of integrin-targeted and cell-penetrating peptides for cancer chemotherapy. Bioconjug Chem. 2018;29(6):1973–2000. https://doi.org/10.1021/acs.bioconjchem.8b00208.
Liyanage PY, Zhou Y, Al-Youbi AO, Bashammakh AS, El-Shahawi MS, Vanni S, Graham RM, Leblanc RM. Pediatric glioblastoma target-specific efficient delivery of gemcitabine across the blood-brain barrier: via carbon nitride dots. Nanoscale. 2020;12(14):7927–38. https://doi.org/10.1039/d0nr01647k.
Di Gioia S, Trapani A, Cassano R, Di Gioia ML, Trombino S, Cellamare S, Bolognino I, Hossain MN, Sanna E, Trapani G, Conese M. Nose-to-brain delivery: a comparative study between carboxymethyl chitosan based conjugates of dopamine. Int J Pharm. 2021;599(February):120453. https://doi.org/10.1016/j.ijpharm.2021.120453.
Singh AK, Gothwal A, Rani S, Rana M, Sharma AK, Yadav AK, Gupta U. Dendrimer donepezil conjugates for improved brain delivery and better in vivo pharmacokinetics. ACS Omega. 2019;4(3):4519–28. https://doi.org/10.1021/acsomega.8b03445.
Kalia J, Raines RT. Hydrolytic stability of hydrazones and oximes. Angew Chemie - Int Ed. 2008;47(39):7523–6. https://doi.org/10.1002/anie.200802651.
Senter PD. Potent antibody drug conjugates for cancer therapy. Curr Opin Chem Biol. 2009;13(3):235–44. https://doi.org/10.1016/j.cbpa.2009.03.023.
Nie W, Wu G, Zhang J, Huang L, Ding J, Jiang A, Zhang Y, Liu Y, Li J, Pu K, Xie H. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angew Chemie. 2020;132(5):2034–8. https://doi.org/10.1002/ange.201912524.
Tao Y, Liu S, Zhang Y, Chi Z, Xu J. A PH-responsive polymer based on dynamic imine bonds as a drug delivery material with pseudo target release behavior. Polym Chem. 2018;9(7):878–84. https://doi.org/10.1039/c7py02108a.
Firestone RA, Willner D, Hofstead SJ, King HD, Kaneko T, Braslawsky GR, Greenfield RS, Trail PA, Lasch SJ, Henderson AJ, Casazza AM, Hellström I, Hellström KE. Synthesis and antitumor activity of the immunoconjugate BR96-Dox. J Control Release. 1996;39(2–3):251–9. https://doi.org/10.1016/0168-3659(95)00160-3.
Schuster S, Biri-Kovács B, Szeder B, Farkas V, Buday L, Szabó Z, Halmos G, Mező G. Synthesis and in vitro biochemical evaluation of oxime bond-linked daunorubicin-GnRH-III conjugates developed for targeted drug delivery. Beilstein J Org Chem. 2018;14:756–71. https://doi.org/10.3762/bjoc.14.64.
Broadwell RD, Balin BJ. Endocytic and exocytic pathways of the neuronal secretory process and trans synaptic transfer of wheat germ agglutinin-horseradish peroxidase in vivo. J Comp Neurol. 1985;242(4):632–50. https://doi.org/10.1002/cne.902420410.
Thorne RG, Emory CR, Ala TA, Frey WH. Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 1995;692(1–2):278–82. https://doi.org/10.1016/0006-8993(95)00637-6.
Gao X, Tao W, Lu W, Zhang Q, Zhang Y, Jiang X, Fu S. Lectin-conjugated PEG-PLA nanoparticles: preparation and brain delivery after intranasal administration. Biomaterials. 2006;27(18):3482–90. https://doi.org/10.1016/j.biomaterials.2006.01.038.
Elzoghby AO, Samy WM, Elgindy NA. Protein-based nanocarriers as promising drug and gene delivery systems. J Control Release. 2012;161(1):38–49. https://doi.org/10.1016/j.jconrel.2012.04.036.
Elzoghby AO, Freag MS, Elkhodairy KA. Biopolymeric nanoparticles for targeted drug delivery to brain tumors. Elsevier Inc., 2018. https://doi.org/10.1016/B978-0-12-812218-1.00007-5.
Sonvico F, Clementino A, Buttini F, Colombo G, Pescina S, Guterres SS, Pohlmann AR, Nicoli S. Surface-modified nanocarriers for nose-to-brain delivery: from bioadhesion to targeting. Pharmaceutics. 2018;10(1):1–34. https://doi.org/10.3390/pharmaceutics10010034.
Piazzini V, Landucci E, D’Ambrosio M, Tiozzo Fasiolo L, Cinci L, Colombo G, Pellegrini-Giampietro DE, Bilia AR, Luceri C, Bergonzi MC. Chitosan coated human serum albumin nanoparticles: a promising strategy for nose-to-brain drug delivery. Int J Biol Macromol. 2019;129:267–80. https://doi.org/10.1016/j.ijbiomac.2019.02.005.
Kulkarni N, Shinde SD, Jadhav GS, Adsare DR, Rao K, Kachhia M, Maingle M, Patil SP, Arya N, Sahu B. Peptide-chitosan engineered scaffolds for biomedical applications. Bioconjug Chem. 2021;32(3):448–65. https://doi.org/10.1021/acs.bioconjchem.1c00014.
Shi S, Zhang L, Zhu M, Wan G, Li C, Zhang J, Wang Y, Wang Y. Reactive oxygen species-responsive nanoparticles based on peglated prodrug for targeted treatment of oral tongue squamous cell carcinoma by combining photodynamic therapy and chemotherapy. ACS Appl Mater Interfaces. 2018;10(35):29260–72. https://doi.org/10.1021/acsami.8b08269.
Qian C, Wang J, Qian Y, Hu R, Zou J, Zhu C, Zhu C, Zhu Y, Qi S, Jia X, Wu L, Li W, Chen Z. Tumor-cell-surface adherable peptide-drug conjugate prodrug nanoparticles inhibit tumor metastasis and augment treatment efficacy. Nano Lett. 2020;20(6):4153–61. https://doi.org/10.1021/acs.nanolett.0c00152.
Yang D, Sun YY, Lin X, Baumann JM, Dunn RS, Lindquist DM, Kuan CY. Intranasal delivery of cell-penetrating anti-NF-ΚB peptides (Tat-NBD) alleviates infection-sensitized hypoxic-ischemic brain injury. Exp Neurol. 2013;247:447–55. https://doi.org/10.1016/j.expneurol.2013.01.015.
Meredith ME, Salameh TS, Banks WA. Intranasal delivery of proteins and peptides in the treatment of neurodegenerative diseases. AAPS J. 2015;17(4):780–7. https://doi.org/10.1208/s12248-015-9719-7.
Cardoso MM, Peca IN, Roque ACA. Antibody-conjugated nanoparticles for therapeutic applications. Curr Med Chem. 2012;19(19):3103–27. https://doi.org/10.2174/092986712800784667.
Pothin E, Lesuisse D, Lafaye P. Brain delivery of single-domain antibodies: a focus on VHH and VNAR. Pharmaceutics. 2020;12(10):1–16. https://doi.org/10.3390/pharmaceutics12100937.
Chu L, Wanga A, Ni L, Yan X, Song Y, Zhao M, Sun K, Mu H, Liu S, Wu Z, Zhang C. Nose-to-brain delivery of temozolomide-loaded Plga nanoparticles functionalized with anti-Epha3 for glioblastoma targeting. Drug Deliv. 2018;25(1):1634–41. https://doi.org/10.1080/10717544.2018.1494226.
Ferreira NN, de Oliveira Junior E, Granja S, Boni FI, Ferreira LMB, Cury BSF, Santos LCR, Reis RM, Lima EM, Baltazar F, Gremião MPD. Nose-to-brain co-delivery of drugs for glioblastoma treatment using nanostructured system. Int J Pharm. 2021. https://doi.org/10.1016/j.ijpharm.2021.120714.
Aderibigbe BA, Naki T. Chitosan-based nanocarriers for nose to brain delivery. Appl Sci. 2019. https://doi.org/10.3390/app9112219.
Ji J, Wang L, Yu H, Chen Y, Zhao Y, Zhang H, Amer WA, Sun Y, Huang L, Saleem M. Chemical modifications of chitosan and its applications. Polym - Plast Technol Eng. 2014;53(14):1494–505. https://doi.org/10.1080/03602559.2014.909486.
Ruby JJ, Pandey VP. Formulation and evaluation of olanzapine loaded chitosan nanoparticles for nose to brain targeting an in vitro and ex vivo toxicity study. J Appl Pharm Sci. 2016;6(9):034–40. https://doi.org/10.7324/japs.2016.60905.
Rukmangathen R, Yallamalli IM, Yalavarthi PR. Formulation and biopharmaceutical evaluation of risperidone-loaded chitosan nanoparticles for intranasal delivery. Drug Dev Ind Pharm. 2019;45(8):1342–50. https://doi.org/10.1080/03639045.2019.1619759.
Patel D, Naik S, Misra A. Improved transnasal transport and brain uptake of tizanidine HCl-loaded thiolated chitosan nanoparticles for alleviation of pain. J Pharm Sci. 2012;101(2):690–706. https://doi.org/10.1002/jps.22780.
Haque S, Sahni JK, Ali J, Baboota S. Development and evaluation of brain targeted intranasal alginate nanoparticles for treatment of depression. J Psychiatr Res. 2014;48(1):1–12. https://doi.org/10.1016/j.jpsychires.2013.10.011.
Kim YS, Sung DK, Kim H, Kong WH, Kim YE, Hahn SK. Nose-to-brain delivery of hyaluronate – FG loop peptide conjugate for non-invasive hypoxic-ischemic encephalopathy therapy. J Control Release. 2018;2019(307):76–89. https://doi.org/10.1016/j.jconrel.2019.06.021.
Pan L, Zhou J, Ju F, Zhu H. Intranasal delivery of α-asarone to the brain with lactoferrin-modified MPEG-PLA nanoparticles prepared by premix membrane emulsification. Drug Deliv Transl Res. 2018;8(1):83–96. https://doi.org/10.1007/s13346-017-0438-8.
Tang S, Wang A, Yan X, Chu L, Yang X, Song Y, Sun K, Yu X, Liu R, Wu Z, Xue P. Brain-targeted intranasal delivery of dopamine with borneol and lactoferrin co-modified nanoparticles for treating Parkinson’s disease. Drug Deliv. 2019;26(1):700–7. https://doi.org/10.1080/10717544.2019.1636420.
Bi CC, Wang AP, Chu YC, Liu S, Mu HJ, Liu WH, Wu ZM, Sun KX, Li YX. Intranasal delivery of rotigotine to the brain with lactoferrin-modified PEG-PLGA nanoparticles for Parkinson’s disease treatment. Int J Nanomedicine. 2016;11:6547–59. https://doi.org/10.2147/IJN.S120939.
Liu Z, Jiang M, Kang T, Miao D, Gu G, Song Q, Yao L, Hu Q, Tu Y, Pang Z, Chen H, Jiang X, Gao X, Chen J. Lactoferrin-modified PEG-Co-PCL nanoparticles for enhanced brain delivery of NAP peptide following intranasal administration. Biomaterials. 2013;34(15):3870–81. https://doi.org/10.1016/j.biomaterials.2013.02.003.
Su Y, Sun B, Gao X, Dong X, Fu L, Zhang Y, Li Z, Wang Y, Jiang H, Han B. Intranasal delivery of targeted nanoparticles loaded with MiR-132 to brain for the treatment of neurodegenerative diseases. Front Pharmacol. 2020;11(August):1–13. https://doi.org/10.3389/fphar.2020.01165.
Alarcón-Arís D, Recasens A, Galofré M, Carballo-Carbajal I, Zacchi N, Ruiz-Bronchal E, Pavia-Collado R, Chica R, Ferrés-Coy A, Santos M, Revilla R, Montefeltro A, Fariñas I, Artigas F, Vila M, Bortolozzi A. Selective α-synuclein knockdown in monoamine neurons by intranasal oligonucleotide delivery: potential therapy for Parkinson’s disease. Mol Ther. 2018;26(2):550–67. https://doi.org/10.1016/j.ymthe.2017.11.015.
Kanazawa T, Taki H, Tanaka K, Takashima Y, Okada H. Cell-penetrating peptide-modified block copolymer micelles promote direct brain delivery via intranasal administration. Pharm Res. 2011;28(9):2130–9. https://doi.org/10.1007/s11095-011-0440-7.
Kanazawa T, Akiyama F, Kakizaki S, Takashima Y, Seta Y. Delivery of SiRNA to the brain using a combination of nose-to-brain delivery and cell-penetrating peptide-modified nano-micelles. Biomaterials. 2013;34(36):9220–6. https://doi.org/10.1016/j.biomaterials.2013.08.036.
Kanazawa T, Morisaki K, Suzuki S, Takashima Y. Prolongation of life in rats with malignant glioma by intranasal SiRNA/drug codelivery to the brain with cell-Penetrating peptide-modified micelles. Mol Pharm. 2014;11(5):1471–8. https://doi.org/10.1021/mp400644e.
Kanazawa T. Brain delivery of small interfering ribonucleic acid and drugs through intranasal administration with nano-sized polymer micelles. Med Devices Evid Res. 2015;8:57–64. https://doi.org/10.2147/MDER.S70856.
Veronesi MC, Alhamami M, Miedema SB, Yun Y, Ruiz-Cardozo M, Vannier MW. Imaging of intranasal drug delivery to the brain. Am J Nucl Med Mol Imaging. 2020;10(1):1–31.
Gao X, Chen J, Chen J, Wu B, Chen H, Jiang X. Quantum dots bearing lectin-functionalized nanoparticles as a platform for in vivo brain imaging. Bioconjug Chem. 2008;19(11):2189–95. https://doi.org/10.1021/bc8002698.
Velasco-Aguirre C, Morales F, Gallardo-Toledo E, Guerrero S, Giralt E, Araya E, Kogan MJ. Peptides and proteins used to enhance gold nanoparticle delivery to the brain: preclinical approaches. Int J Nanomedicine. 2015;10:4919–36. https://doi.org/10.2147/IJN.S82310.
Sukumar UK, Bose RJC, Malhotra M, Babikir HA, Afjei R, Robinson E, Zeng Y, Chang E, Habte F, Sinclair R, Gambhir SS, Massoud TF, Paulmurugan R. Intranasal delivery of targeted polyfunctional gold–iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide. Biomaterials. 2019;218(March):119342. https://doi.org/10.1016/j.biomaterials.2019.119342.
Rajora MA, Ding L, Valic M, Jiang W, Overchuk M, Chen J, Zheng G. Tailored theranostic apolipoprotein E3 porphyrin-lipid nanoparticles target glioblastoma. Chem Sci. 2017;8(8):5371–84. https://doi.org/10.1039/c7sc00732a.
Khan AR, Liu M, Khan MW, Zhai G. Progress in brain targeting drug delivery system by nasal route. J Control Release. 2017;268(September):364–89. https://doi.org/10.1016/j.jconrel.2017.09.001.
Richards DA, Thomas MR, Szijj PA, Foote J, Chen Y, Nogueira JCF, Chudasama V, Stevens MM. Employing defined bioconjugates to generate chemically functionalised gold nanoparticles for: in vitro diagnostic applications. Nanoscale. 2021;13(27):11921–31. https://doi.org/10.1039/d1nr02584h.
Díaz-González M, de la Escosura-Muñiz A, Fernandez-Argüelles MT, García Alonso FJ, Costa-Fernandez JM. Quantum dot bioconjugates for diagnostic applications. Springer International Publishing; 2020. Vol. 378. https://doi.org/10.1007/s41061-020-0296-6.
Acknowledgements
The authors are grateful to the National Institute of Pharmaceutical Education and Research (NIPER), Ahmedabad, and highly acknowledged to former Director, Prof. Kiran Kalia for the support.
Funding
BS and GJ would like to acknowledge the Department of Science and Technology (DST)-Science and Engineering Research Board (SERB) for providing the grant (file no. SRG/2020/001241).
Author information
Authors and Affiliations
Contributions
TA and GJ drafted the manuscript; BS contributed in giving valuable suggestion for bioconjugation portion written.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors do hereby give their consent for publication of the work entitled “Recent Trends of Bioconjugated Nanomedicines through Nose-to-Brain Delivery for Neurological Disorders” in drug delivery and translational research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Articles drafting and selection criteria
The article proposed here has been drafted following the given steps: we searched PubMed, Science Direct, and SciFinder (1983–2021) with the search terms “nose-to-brain delivery,” “bioconjugation types and strategies,” “nanoparticles for the nose-to-brain delivery,” and “bioconjugated nanosystems.” We primarily selected articles from the last 10 years and also included older publications that are found to be establishing the foundation for the concepts outlined in the manuscript.
Rights and permissions
About this article
Cite this article
Agnihotri, T.G., Jadhav, G.S., Sahu, B. et al. Recent trends of bioconjugated nanomedicines through nose-to-brain delivery for neurological disorders. Drug Deliv. and Transl. Res. 12, 3104–3120 (2022). https://doi.org/10.1007/s13346-022-01173-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01173-y