Abstract
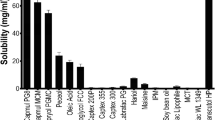
Raloxifene (RLX) is a drug that is commonly recommended to postmenopausal women at high risk of invasive breast cancer and to prevent osteoporosis. However, limited water solubility (0.000512 mg/ml) and low oral bioavailability (2%) of RLX limit its therapeutic utility. The objective of the present study was to develop an alternative transdermal delivery of RLX to improve its absorption, bypass first pass metabolism, and subsequently improve bioavailability. RLX-loaded cubosomes were prepared using the ethanol injection method followed by microfluidization technique and optimized using the QbD-based 23 factorial design. The average particle size, entrapment efficiency, and zeta potential of the optimized formulation were found to be 110.6 nm, 98.23%, and 26.2 mV, respectively. In vitro dissolution study indicated that the RLX-loaded cubosomes released 98.26% of the drug compared to pure RLX dispersion (58.6%). Histopathological examination revealed no sign of inflammation, indicating the safety of the developed formulation. Accelerated stability study as per ICH guidelines displayed no significant change in the formulation characteristics and drug-related performance of the developed formulation. Ex vivo permeability studies demonstrated a prolonged release from cubosomal formulation. In vivo pharmacokinetic studies revealed that the relative bioavailability of the optimized transdermal RLX-loaded cubosomes increased by 2.33-fold and 1.22-fold when compared with the oral RLX dispersion and transdermal RLX hydro-ethanolic solution respectively. IVIVC showed level C correlation with linear regression. Thus, the developed RLX-loaded cubosomes may have potential to overcome the problems associated with the existing marketed oral dosage forms of RLX.
Graphical abstract








Similar content being viewed by others
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin Wiley. 2018;68:394–424.
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. Wiley-Liss Inc. 2019;144:1941–53.
Organization WH. Cancer Today [Internet]. Int. Agency Res. 2018;2. Available from: https://gco.iarc.fr/today/fact-sheets-populations/0A.
Medicine N. library of Raloxifene hydrochloride C28H28ClNO4S - PubChem [Internet]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Raloxifene-hydrochloride. Accessed Mar 2022.
Tran TH, Poudel BK, Marasini N, Chi SC, Choi HG, Yong CS, et al. Preparation and evaluation of raloxifene-loaded solid dispersion nanoparticle by spray-drying technique without an organic solvent. Int J Pharm. Elsevier B.V. 2013;443:50–7.
Elsheikh MA, Elnaggar YSR, Gohar EY, Abdallah OY. Nanoemulsion liquid preconcentrates for raloxifene hydrochloride: Optimization and in vivo appraisal. Int J Nanomedicine. 2012.
Thakkar H, Nangesh J, Parmar M, Patel D. Formulation and characterization of lipid-based drug delivery system of raloxifene-microemulsion and self-microemulsifying drug delivery system. J Pharm Bioallied Sci. 2011;3:442–8.
Ağardan NBM, Değim Z, Yılmaz, Altıntaş L, Topal T. Tamoxifen/raloxifene loaded liposomes for oral treatment of breast cancer. J Drug Deliv Sci Technol. 2020.
Hosny KM, Bahmdan RH, Alhakamy NA, Alfaleh MA, Ahmed OA, Elkomy MH. Physically optimized nano-lipid carriers augment raloxifene and vitamin D oral bioavailability in healthy humans for management of osteoporosis. J Pharm Sci. Elsevier Ltd. 2020.
Fontana MC, Laureano JV, Forgearini B, dos Santos J, Pohlmann AR, Guterres SS, et al. Spray-dried raloxifene submicron particles for pulmonary delivery: development and in vivo pharmacokinetic evaluation in rats. Int J Pharm. 2020;585.
Ravi PR, Aditya N, Kathuria H, Malekar S, Vats R. Lipid nanoparticles for oral delivery of raloxifene: optimization, stability, in vivo evaluation and uptake mechanism. Eur J Pharm Biopharm. Elsevier B.V. 2014.
Nagai N, Ogata F, Otake H, Nakazawa Y, Kawasaki N. Design of a transdermal formulation containing raloxifene nanoparticles for osteoporosis treatment. Int J Nanomedicine. 2018;13:5215–29.
Ağardan NBM, Değim Z, Yılmaz, Altıntaş L, Topal T. Tamoxifen/raloxifene loaded liposomes for oral treatment of breast cancer. J Drug Deliv Sci Technol. 2020;57.
Waheed A, Aqil M, Ahad A, Imam SS, Moolakkadath T, Iqbal Z, et al. Improved bioavailability of raloxifene hydrochloride using limonene containing transdermal nano-sized vesicles. J Drug Deliv Sci Technol Elsevier. 2019;52:468–76.
Kong Y, Cai H, Xing H, Ren C, Kong D, Ning C, et al. Pulmonary delivery alters the disposition of raloxifene in rats. J Pharm Pharmacol. 2020.
Burra M, Jukanti R, Janga KY, Sunkavalli S, Velpula A, Ampati S, et al. Enhanced intestinal absorption and bioavailability of raloxifene hydrochloride via lyophilized solid lipid nanoparticles. Adv Powder Technol. 2013;24:393–402.
Abdel-Salam FS, Elkheshen SA, Mahmoud AA, Basalious EB, Amer MS, Mostafa AA, et al. In-situ forming chitosan implant-loaded with raloxifene hydrochloride and bioactive glass nanoparticles for treatment of bone injuries: formulation and biological evaluation in animal model. Int J Pharm. Elsevier B.V. 2020.
Kamel R, El-Wakil NA, Abdelkhalek AFA, Elkasabgy NA. Nanofibrillated cellulose/cyclodextrin based 3D scaffolds loaded with raloxifene hydrochloride for bone regeneration. Int J Biol Macromol. Elsevier B.V. 2020.
Elkasabgy NA, Abdel-Salam FS, Mahmoud AA, Basalious EB, Amer MS, Mostafa AA, et al. Long lasting in-situ forming implant loaded with raloxifene HCl: an injectable delivery system for treatment of bone injuries. Int J Pharm. Elsevier B.V. 2019.
Cheraghi S, Taher MA, Karimi-Maleh H. A sensitive amplified sensor based on improved carbon paste electrode with 1-methyl-3-octylimidazolium tetrafluoroborate and ZnO/CNTs nanocomposite for differential pulse voltammetric analysis of raloxifene. Appl Surf Sci. Elsevier B.V. 2017.
Kanade R, Boche M, Pokharkar V. Self-assembling raloxifene loaded mixed micelles: formulation optimization, in vitro cytotoxicity and in vivo pharmacokinetics. AAPS PharmSciTech. 2018.
Larsson K. Cubic lipid-water phases: structures and biomembrane aspects. J Phys Chem American Chemical Society. 1989;93:7304–14.
Yang D, O’Brien DF, Marder SR. Polymerized bicontinuous cubic nanoparticles (cubosomes) from a reactive monoacylglycerol. J Am Chem Soc. 2002;124:13388–9.
Murgia S, Biffi S, Mezzenga R. Recent advances of non-lamellar lyotropic liquid crystalline nanoparticles in nanomedicine. Curr Opin Colloid Interface Sci. Elsevier Ltd. 2020;48:28–39.
Madheswaran T, Kandasamy M, Bose RJ, Karuppagounder V. Current potential and challenges in the advances of liquid crystalline nanoparticles as drug delivery systems. Drug Discov Today Elsevier Ltd. 2019;24:1405–12.
Spicer PT. Progress in liquid crystalline dispersions: cubosomes. Curr Opin Colloid Interface Sci. 2005;10:274–9.
Garg G, Saraf S. Cubosomes: an overview. Biol Pharm Bull. 2007.
Sadhu VR, Beram NS, Kantamneni P. A review on cubosome: the novel drug delivery system. GSC Biol Pharm Sci. 2018;5:076–81.
Tilekar K, Khade P, Kakade S, Kotwal S, Patil R. Cubosomes-a drug delivery system. Int J Pharm Chem Biol Sci. 2014;4:812–24.
Spicer P. Cubosome processing: industrial nanoparticle technology development. Chem Eng Res Des. 2005;83:1283–6.
Yingchoncharoen P, Kalinowski DS, Richardson DR. Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharmacol Rev. 2016;68:701–87.
Article R. A review on cubosome: a novel approach for drug delivery A. R. Tekade and G. D. Avhad Department of Pharmaceutics, Marathwada Mitra Mandal's College of Pharmacy (MMCOP), Thergaon, Pune - 411033, Maharashtra, India. 2022;13:579–88.
Karami Z, Hamidi M. Cubosomes : remarkable drug delivery potential. Drug Discov Today Elsevier Ltd. 2016;21:789–801.
Naveentaj S, Muzib YI. A review on liquid crystalline nanoparticles (cubosomes): emerging nanoparticulate drug carrier. Int J Curr Pharm Res. 2020.
Gaballa S, El Garhy O, Abdelkader H. Cubosomes: composition, preparation, and drug delivery applications. J Adv Biomed Pharm Sci. 2019;0:0–0.
Anbarasan B, Grace XF, Shanmuganathan S. An overview of cubosomes - smart drug delivery system. Sri Ramachandra J Med. 2015;8:1–4.
Daware SU, Saudagar RB. Formulation and development of cubosome loaded emulgel - a review. 2017;10:918–24.
Gaballa SA, El Garhy OH, Moharram H, Abdelkader H. Preparation and evaluation of cubosomes/cubosomal gels for ocular delivery of beclomethasone dipropionate for management of uveitis. Pharm Res. 2020;37.
Chacko IA, Ghate VM, Dsouza L, Lewis SA. Colloids and surfaces B: biointerfaces lipid vesicles: a versatile drug delivery platform for dermal and transdermal applications. Colloids Surfaces B Biointerfaces: Elsevier. 2020.
Patil SS, Mahadik KR, Paradkar AR. Liquid crystalline phase as a probe for crystal engineering of lactose: carrier for pulmonary drug delivery. Eur J Pharm Sci. Elsevier B.V. 2015;68:43–50.
Boge L, Umerska A, Matougui N, Bysell H, Ringstad L, Davoudi M, et al. Cubosomes post-loaded with antimicrobial peptides: characterization, bactericidal effect and proteolytic stability. Int J Pharm. Elsevier B.V. 2017.
Ravikumar R, Ganesh M, Senthil V, Ramesh YV, Jakki SL, Choi EY. Tetrahydro curcumin loaded PCL-PEG electrospun transdermal nanofiber patch: preparation, characterization, and in vitro diffusion evaluations. J Drug Deliv Sci Technol. 2018;44:342–8.
Salamanca CH, Barrera-Ocampo A, Lasso JC, Camacho N, Yarce CJ. Franz diffusion cell approach for pre-formulation characterisation of ketoprofen semi-solid dosage forms. Pharmaceutics. 2018;10:1–10.
Rarokar NR, Saoji SD, Raut NA, Taksande JB, Khedekar PB, Dave VS. Nanostructured cubosomes in a thermoresponsive depot system: an alternative approach for the controlled delivery of docetaxel. AAPS PharmSciTech. 2016;17:436–45.
Da DY, Larson I, Barnes TJ, Prestidge CA, Allen S, Chen X, et al. Understanding the interfacial properties of nanostructured liquid crystalline materials for surface-specific delivery applications. Langmuir. 2012;28:13485–95.
Naidjonoka P, Fornasier M, Pålsson D, Rudolph G, Al-Rudainy B, Murgia S, et al. Bicontinuous cubic liquid crystalline phase nanoparticles stabilized by softwood hemicellulose. Colloids Surfaces B Biointerfaces. 2021.
Castro RD. Production and characterization of cationic cubosomes. 2019.
Rajabalaya R, Musa MN, Kifli N, David SR. Oral and transdermal drug delivery systems: Role of lipid-based lyotropic liquid crystals. Drug Des Devel Ther. 2017;11:393–406.
Noor AH, Ghareeb MM. Formulation and evaluation of ondansetron HCl nanoparticles for transdermal delivery. Iraqi J Pharm Sci Drug Delivery and Translational Research. 2020.
Acknowledgements
The authors thank Cipla Pvt. Ltd. (Pune, India) for providing us the gift sample of RLX. The authors are much obliged to Danisco India Private Limited (Mumbai, India) and Gattefossé S.A.S. (Saint Priest, France) for providing GMO and Pluronic F127. The authors like to present their gratitude to Regional Ayurveda Institute of Research for their support, facility, and guidance for histopathological studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This animal study was approved by the Committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEA). CPCSEA protocol number: 1703/PO/Re/S/01/CPCSEA.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Human and animal rights
No humans were used for the studies that are basis of this research. The animal studies were performed strictly in accordance with institutional and CPCSEA guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gupta, T., Kenjale, P. & Pokharkar, V. QbD-based optimization of raloxifene-loaded cubosomal formulation for transdemal delivery: ex vivo permeability and in vivo pharmacokinetic studies. Drug Deliv. and Transl. Res. 12, 2979–2992 (2022). https://doi.org/10.1007/s13346-022-01162-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01162-1