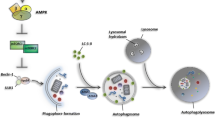
Abstract
Autophagy is a catabolic process in which an organism responds to its nutrient or metabolic emergencies. It involves the degradation of cytoplasmic proteins and organelles by forming double-membrane vesicles called “autophagosomes.” They sequester cargoes, leading them to degradation in the lysosomes. Although autophagy acts as a protective mechanism for maintaining homeostasis through cellular recycling, it is ostensibly a cause of certain cancers, but a cure for others. In other words, insufficient autophagy, due to genetic or cellular dysfunctions, can lead to tumorigenesis. However, many autophagy modulators are developed for cancer therapy. Diverse nanoparticles have been documented to induce autophagy. Also, the highly stable nanoparticles show blockage to autophagic flux. In this review, we revealed a general mechanism by which autophagy can be induced or blocked via nanoparticles as well as several studies recently performed to prove the stated fact. In addition, we have also elucidated the paradoxical roles of autophagy in cancer and how their differential role at different stages of various cancers can affect its treatment outcomes. And finally, we summarize the breakthroughs in cancer disease treatments by using metallic, polymeric, and liposomal nanoparticles as potent autophagy modulators.
Graphical abstract





Similar content being viewed by others
Data availability
Not applicable.
References
Bhattacharya S, Patel KK, Dehari D, Agrawal AK, Singh S. Melatonin and its ubiquitous anticancer effects. Springer US. Mol Cell Biochem [Internet]. 2019;462:133–55. https://doi.org/10.1007/s11010-019-03617-5.
Aqil F, Jeyabalan J, Agrawal AK, Kyakulaga AH, Munagala R, Parker L, et al. Exosomal delivery of berry anthocyanidins for the management of ovarian cancer. Food Funct Royal. 2017;8:4100–7.
Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW, et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine Nanotechnology. Elsevier Inc. Biol Med [Internet]. 2017;13:1627–36. https://doi.org/10.1016/j.nano.2017.03.001.
Munagala R, Aqil F, Jeyabalan J, Agrawal AK, Mudd M, Kyakulaga AH, et al. Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett. 2018;393:94–102.
Jain S, Jain R, Das M, Agrawal AK, Thanki K, Kushwah V. Combinatorial bio-conjugation of gemcitabine and curcumin enables dual drug delivery with synergistic anticancer efficacy and reduced toxicity. RSC Adv. 2014;4:29193–201.
Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100:57–70.
Crighton D, Ryan KM. Splicing DNA-damage responses to tumour cell death. Biochim Biophys Acta- Rev Cancer. 2004;1705:3–15.
Aita VM, Liang XH, Murty VVVS, Pincus DL, Yu W, Cayanis E, et al. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59:59–65.
Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin1. Nature. 1999;402:672–6.
Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BH, et al. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol. 2006;8:688–98.
Knævelsrud H, Ahlquist T, Merok MA, Nesbakken A, Stenmark H, Lothe RA, et al. UVRAG mutations associated with microsatellite unstable colon cancer do not affect autophagy. Autophagy. 2010;6:863–70.
Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–20.
Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA. 2003;100:15077–82.
Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sato Y, Liang C, et al. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol. 2007;9:1142–51.
Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64.
Yun CW, Lee SH. The roles of autophagy in cancer. Int J Mol Sci. 2018;19:1–18.
Rosenfeldt MT, Ryan KM. The multiple roles of autophagy in cancer. Carcinogenesis. 2011;32:955–63.
Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, et al. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–35.
Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21:1367–81.
Miracco C, Cosci E, Oliveri G, Luzi P, Pacenti L, Monciatti I, et al. Protein and mRNA expression of autophagy gene Beclin 1 in human brain tumours. Int J Oncol. 2007;30:429–36.
Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25:795–800.
Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–34.
Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–6.
Mariño G, Salvador-Montoliu N, Fueyo A, Knecht E, Mizushima N, López-Otín C. Tissue-specific autophagy alterations and increased tumorigenesis in mice deficient in Atg4C/autophagin-3. J Biol Chem. 2019;282:18573–83.
Wen ZP, Zeng WJ, Chen YH, Li H, Wang JY, Cheng Q, et al. Knockdown ATG4C inhibits gliomas progression and promotes temozolomide chemosensitivity by suppressing autophagic flux. J Exp Clin Cancer Res. 2019;38:1–15.
Kim MS, Jeong EG, Ahn CH, Kim SS, Lee SH, Yoo NJ. Frameshift mutation of UVRAG, an autophagy-related gene, in gastric carcinomas with microsatellite instability. Hum Pathol. 2008;39:1059–63.
He S, Zhao Z, Yang Y, O’Connell D, Zhang X, Oh S, et al. Truncating mutation in the autophagy gene UVRAG confers oncogenic properties and chemosensitivity in colorectal cancers. Nat Commun. 2015;6:1–14.
Zhao Z, Oh S, Li D, Ni D, Pirooz SD, Lee J-H, et al. A Dual Role for UVRAG in Maintaining Chromosomal Stability Independent of Autophagy. Dev Cell. 2012;22:1001–16.
Moscat J, Diaz-Meco MT. p62 at the Crossroads of Autophagy, Apoptosis, and Cancer. Cell. 2009;137:1001–4.
Taniguchi K, Yamachika S, He F, Karin M. p62/SQSTM1- Dr. Jekyll and Mr. Hyde that prevents oxidative stress but promotes liver cancer. FEBS Lett. 2016;590:2375–97.
Liu B, Fang M, He Z, Cui D, Jia S, Lin X, et al. Hepatitis B virus stimulates G6PD expression through HBx-mediated Nrf2 activation. Cell Death Dis. 2015;6:1–10.
Thomasina Barron E, Smyth PPA, McDermott EW, Tobbia IN, Higgins NJO. Quantitative cytochemistry of glucose-6-phosphate dehydrogenase in benign and malignant breast tumours. Eur J Cancer Clin Oncol. 1991;27:985–9.
Chen Z, Wang J, Yuan W, Chen Z, Wu S, Chen J, et al. Overexpression of G6PD is associated with poor clinical outcome in gastric cancer. Tumor Biol. 2012;33:95–101.
Zampella EJ, Ii TGP. Glucose-6-Phosphate Dehydrogenase : A Possible Clinical lndica tor for Prosta tic Carcinoma. Cancer. 1982;49:384–7.
Young AR, Narita M, Ferreira M, Kirschner K, Sadaie M, Darot JF, et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23:798–803.
Ahn CH, Jeong EG, Lee JW, Kim MS, Kim SH, Kim SS, et al. Expression of beclin-1, an autophagy-related protein, in gastric and colorectal cancers. APMIS. 2007;115:1344–9.
Parkhitko A, Myachina F, Morrison TA, Hindi KM, Auricchio N, Karbowniczek M, et al. Tumorigenesis in tuberous sclerosis complex is autophagy and p62/sequestosome 1 (SQSTM1)-dependent. Proc Natl Acad Sci USA. 2011;108:12455–60.
Valentin-Vega YA, Kastan MB. A new role for ATM: Regulating mitochondrial function and mitophagy. Autophagy. 2012;8:840–1.
Wei H, Wei S, Gan B, Peng X, Zou W, Guan JL. Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis. Genes Dev. 2011;25:1510–27.
Kim MJ, Woo SJ, Yoon CH, Lee JS, An S, Choi YH, et al. Involvement of autophagy in oncogenic K-Ras-induced malignant cell transformation. J Biol Chem. 2011;286:12924–32.
Izuishi K, Kato K, Ogura T, Kinoshita T, Esumi H. Remarkable tolerance of tumor cells to nutrient deprivation: Possible new biochemical target for cancer therapy. Cancer Res. 2000;60:6201–7.
Mathew R, Karantza-Wadsworth V, White E. Role of Autophagy in Cancer. Nat Rev Cancer. 2007;7:961–7.
Harris AL. Hypoxia - A key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47.
Lorin S, Hamaï A, Mehrpour M, Codogno P. Autophagy regulation and its role in cancer. Elsevier Ltd. Semin Cancer Biol [Internet]. 2013;23:361–79. https://doi.org/10.1016/j.semcancer.2013.06.007.
Endo H, Inoue M. Dormancy in cancer. Cancer Sci. 2019;110:474–80.
Vera-Ramirez L, Vodnala SK, Nini R, Hunter KW, Green JE. Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence. Springer US. Nat Commun [Internet]. 2018;9. https://doi.org/10.1038/s41467-018-04070-6.
Lu Z, Luo RZ, Lu Y, Zhang X, Yu Q, Khare S, et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J Clin Invest. 2008;118:3917–29.
Amaravadi RK. Autophagy-induced tumor dormancy in ovarian cancer. J Clin Invest. 2008;118:3837–40.
Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Elsevier B.V. Biochim Biophys Acta [Internet]. 2013;1833:3481–98. https://doi.org/10.1016/j.bbamcr.2013.06.026.
Fung C, Lock R, Gao S, Salas E, Debnath J. Induction of Autophagy during Extracellular Matrix Detachment Promotes Cell Survival. Mol Biol Cell. 2008;19:797–806.
Kenific C, Thorburn A, Debnath J. Autophagy and Metastasis: Another double-edged sword. Curr Opin Cell Biol. 2010;22:241–5.
Yang J, Zheng Z, Yan X, Li X, Liu Z, Ma Z. Integration of autophagy and anoikis resistance in solid tumors. Anat Rec. 2013;296:1501–8.
Giatromanolaki AN, St Charitoudis G, Bechrakis NE, Kozobolis VP, Koukourakis MI, Foerster MH, et al. Autophagy patterns and prognosis in uveal melanomas. Mod Pathol Nature Publishing Group. 2011;24:1036–45.
Ding Z Bin, Shi YH, Zhou J, Qiu SJ, Xu Y, Dai Z, et al. Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res. 2008;68:9167–75.
Jang M, Kim SS, Lee J. Cancer cell metabolism: Implications for therapeutic targets. Nature Publishing Group. Exp Mol Med. 2013;45:1–8.
Heiden MGV, Cantley LC, Thompson CB. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The Biology of Cancer: Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metab. 2008;7:11–20.
Eng CH, Abraham RT. The autophagy conundrum in cancer: Influence of tumorigenic metabolic reprogramming. Nature Publishing Group. Oncogene [Internet]. 2011;30:4687–96. https://doi.org/10.1038/onc.2011.220.
Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72.
Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25:460–70.
Lock R, Roy S, Kenific CM, Su JS, Salas E, Ronen SM, et al. Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation. Mol Biol Cell. 2011;22:165–78.
Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–29.
Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res. 2009;15:6479–83.
Manning BD, Cantley LC. AKT/PKB Signaling: Navigating Downstream. Cell. 2007;129:1261–74.
Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–9.
Shortt J, Johnstone RW. Oncogenes in cell survival and cell death. Cold Spring Harb Perspect Biol. 2012;4:1–10.
Nazio F, Bordi M, Cianfanelli V, Locatelli F, Cecconi F. Autophagy and cancer stem cells: molecular mechanisms and therapeutic applications. Springer US. Cell Death Differ [Internet]. 2019;26:690–702. https://doi.org/10.1038/s41418-019-0292-y.
Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer Stem Cells. Int J Biochem Cell Biol. 2012;44:2144–51.
Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–99.
Maycotte P, Jones KL, Goodall ML, Thorburn J, Thorburn A. Autophagy Supports Breast Cancer Stem Cell Maintenance by Regulating IL6 Secretion. Mol Cancer Res. 2015;13:651–8.
Han Y, Fan S, Qin T, Yang J, Sun Y, Lu Y, et al. Role of autophagy in breast cancer and breast cancer stem cells (Review). Int J Oncol. 2018;52:1057–70.
Gong C, Song E, Codogno P, Mehrpour M. The roles of BECN1 and autophagy in cancer are context dependent. Autophagy. 2012;8:1853–5.
Wolf J, Dewi DL, Fredebohm J, Müller-Decker K, Flechtenmacher C, Hoheisel JD, et al. A mammosphere formation RNAi screen reveals that ATG4A promotes a breast cancer stem-like phenotype. Breast Cancer Res. 2013;15:1–13.
Chen X, He Y, Lu F. Autophagy in stem cell biology: A perspective on stem cell self-renewal and differentiation. Hindawi. Stem Cells Int. 2018;2018.
Gong C, Bauvy C, Tonelli G, Yue W, Deloménie C, Nicolas V, et al. Beclin 1 and autophagy are required for the tumorigenicity of breast cancer stem-like/progenitor cells. Oncogene. 2013;32:2261–72.
Chaterjee M, van Golen KL. Breast Cancer Stem Cells Survive Periods of Farnesyl-Transferase Inhibitor-Induced Dormancy by Undergoing Autophagy. Bone Marrow Res. 2011;2011:1–7.
Song Y jiao, Zhang S shan, Guo X ling, Sun K, Han Z peng, Li R, et al. Autophagy contributes to the survival of CD133+ liver cancer stem cells in the hypoxic and nutrient-deprived tumor microenvironment. Elsevier Ireland Ltd. Cancer Lett [Internet]. 2013;339:70–81. https://doi.org/10.1016/j.canlet.2013.07.021.
Zhang D, Zhao Q, Sun H, Yin L, Wu J, Xu J, et al. Defective autophagy leads to the suppression of stem-like features of CD271+ osteosarcoma cells. J Biomed Sci [Internet]. 2016;23:1–12. https://doi.org/10.1186/s12929-016-0297-5.
Peng Q, Qin J, Zhang Y, Cheng X, Wang X, Lu W, et al. Autophagy maintains the stemness of ovarian cancer stem cells by FOXA2. J Exp Clin Cancer Res. 2017;36:1–12.
Buccarelli M, Marconi M, Pacioni S, De Pasqualis I, D’Alessandris QG, Martini M, et al. Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Springer US. Cell Death Dis [Internet]. 2018;9:1–17. https://doi.org/10.1038/s41419-018-0864-7.
Sun K, Deng W, Zhang S, Cai N, Jiao S, Song J, et al. Paradoxical roles of autophagy in different stages of tumorigenesis: Protector for normal or cancer cells. Cell Biosci [Internet]. Cell & Bioscience; 2013;3:1.
Zou Z, Yuan Z, Zhang Q, Long Z, Chen J, Tang Z, et al. Aurora kinase A inhibition-induced autophagy triggers drug resistance in breast cancer cells. Autophagy. 2012;8:1798–810.
Hu YL, Jahangiri A, DeLay M, Aghi MK. Tumor cell autophagy as an adaptive response mediating resistance to treatments such as antiangiogenic therapy. Cancer Res. 2012;72:4294–9.
Sotelo J, Briceño E, López-González MA. Adding Chloroquine to Conventional Treatment for Glioblastoma Multiforme. Ann Intern Med. 2006;144:337.
Li J, Hou N, Faried A, Tsutsumi S, Kuwano H. Inhibition of autophagy augments 5-fluorouracil chemotherapy in human colon cancer in vitro and in vivo model. Elsevier Ltd. Eur J Cancer [Internet]. 2010;46:1900–9. https://doi.org/10.1016/j.ejca.2010.02.021.
Sasaki K, Tsuno NH, Sunami E, Kawai K, Hongo K, Hiyoshi M, et al. Resistance of colon cancer to 5-fluorouracil may be overcome by combination with chloroquine, an in vivo study. Anticancer Drugs. 2012;23:675–82.
Guo XL, Li D, Sun K, Wang J, Liu Y, Song JR, et al. Inhibition of autophagy enhances anticancer effects of bevacizumab in hepatocarcinoma. J Mol Med. 2013;91:473–83.
Shi YH, Ding Z Bin, Zhou J, Hui B, Shi GM, Ke AW, et al. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy. 2011;7:1159–72.
Han W, Pan H, Chen Y, Sun J, Wang Y, Li J, et al. EGFR tyrosine kinase inhibitors activate autophagy as a cytoprotective response in human lung cancer cells. PLoS One. 2011;6:1–8.
Carew JS, Nawrocki ST, Kahue CN, Zhang H, Yang C, Chung L, et al. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHAto overcome Bcr-Abl-mediated drug resistance. Blood. 2007;110:313–22.
Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, et al. Autophagy and chemotherapy resistance: A promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:1–12.
Shimgu T, Fujiwara K, Bogler O, Akiyama Y, Meritake K, Shinojima N, et al. Inhibition of autophagy at a late stage enhances imatinib-induced cytotoxicity In human malignant glioma cells. Int J Cancer. 2009;124:1060–71.
Liu F, Liu D, Yang Y, Zhao S. Effect of autophagy inhibition on chemotherapy-induced apoptosis in A549 lung cancer cells. Oncol Lett. 2013;5:1261–5.
Cao X, Liu B, Cao W, Zhang W, Zhang F, Zhao H, et al. Autophagy inhibition enhances apigenin-induced apoptosis in human breast cancer cells. Chinese J Cancer Res. 2013;25:212–22.
Mujumdar N, Saluja AK. Autophagy in pancreatic cancer: An emerging mechanism of cell death. Autophagy. 2010;6:997–8.
Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, et al. Role of Bcl-2 family proteins in a non-apoptopic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–8.
Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, et al. Regulation of an ATG7–beclin 1 Program of Autophagic Cell Death by Caspase-8. Science. 2004;304:1500–2.
Xiong H yan, Guo X ling, Bu X xin, Zhang S shan, Ma N nan, Song J rui, et al. Autophagic cell death induced by 5-FU in Bax or PUMA deficient human colon cancer cell. Elsevier Ireland Ltd. Cancer Lett [Internet]. 2010;288:68–74. https://doi.org/10.1016/j.canlet.2009.06.039.
Salazar M, Carracedo A, Salanueva ÍJ, Hernández-Tiedra S, Lorente M, Egia A, et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest. 2009;119:1359–72.
Donadelli M, Dando I, Zaniboni T, Costanzo C, Dalla Pozza E, Scupoli MT, et al. Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Nature Publishing Group. Cell Death Dis [Internet]. 2011;2:1–12. https://doi.org/10.1038/cddis.2011.36.
Vara D, Salazar M, Olea-Herrero N, Guzmán M, Velasco G, Díaz-Laviada I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: Role of AMPK-dependent activation of autophagy. Cell Death Differ. 2011;18:1099–111.
Ren SX, Shen J, Cheng ASL, Lu L, Chan RLY, Li ZJ, et al. FK-16 Derived from the Anticancer Peptide LL-37 Induces Caspase-Independent Apoptosis and Autophagic Cell Death in Colon Cancer Cells. PLoS ONE. 2013;8:1–9.
Josset E, Burckel H, Noël G, Bischoff P. The mTOR inhibitor RAD001 potentiates autophagic cell death induced by temozolomide in a glioblastoma cell line. Anticancer Res. 2013;33:1845–51.
Cirone M, Gilardini Montani MS, Granato M, Garufi A, Faggioni A, D’Orazi G. Autophagy manipulation as a strategy for efficient anticancer therapies: Possible consequences. J Exp Clin Cancer Res. 2019;38:1–7.
Sinha R, Kim GJ, Nie S, Shin DM. Nanotechnology in cancer therapeutics: Bioconjugated nanoparticles for drug delivery. Mol Cancer Ther. 2006;5:1909–17.
Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. Elsevier B.V. J Control Release [Internet]. 2015;200:138–57. https://doi.org/10.1016/j.jconrel.2014.12.030.
Gade S, Patel KK, Gupta C, Anjum MM, Deepika D, Agrawal AK, et al. An Ex Vivo Evaluation of Moxifloxacin Nanostructured Lipid Carrier Enriched In Situ Gel for Transcorneal Permeation on Goat Cornea. Elsevier Ltd. J Pharm Sci [Internet]. 2019;108:2905–16. https://doi.org/10.1016/j.xphs.2019.04.005.
Patel KK, Gade S, Anjum MM, Singh SK, Maiti P, Agrawal AK, et al. Effect of penetration enhancers and amorphization on transdermal permeation flux of raloxifene-encapsulated solid lipid nanoparticlean ex vivo study on human skin. Springer International Publishing. Appl Nanosci [Internet]. 2019;9:1383–94. https://doi.org/10.1007/s13204-019-01004-6.
Aqil F, Munagala R, Jeyabalan J, Agrawal AK, Kyakulaga AH, Wilcher SA, et al. Milk exosomes - Natural nanoparticles for siRNA delivery. Elsevier. Cancer Lett [Internet]. 2019;449:186–95. https://doi.org/10.1016/j.canlet.2019.02.011.
Aqil F, Munagala R, Jeyabalan J, Agrawal AK, Gupta R. Exosomes for the Enhanced Tissue Bioavailability and Efficacy of Curcumin. AAPS J The AAPS Journal. 2017;19:1691–702.
Carvajal-Vidal P, González-Pizarro R, Araya C, Espina M, Halbaut L, Gómez de Aranda I, et al. Nanostructured lipid carriers loaded with Halobetasol propionate for topical treatment of inflammation: Development, characterization, biopharmaceutical behavior and therapeutic efficacy of gel dosage forms. Elsevier B.V. Int J Pharm [Internet]. 2020;585:119480. https://doi.org/10.1016/j.ijpharm.2020.119480.
Patel KK, Agrawal AK, Anjum MM, Tripathi M, Pandey N, Bhattacharya S, et al. DNase-I functionalization of ciprofloxacin-loaded chitosan nanoparticles overcomes the biofilm-mediated resistance of Pseudomonas aeruginosa. Springer International Publishing. Appl Nanosci [Internet]. 2020;10:563–75. https://doi.org/10.1007/s13204-019-01129-8.
Patel KK, Surekha DB, Tripathi M, Anjum MM, Muthu MS, Tilak R, et al. Antibiofilm Potential of Silver Sulfadiazine-Loaded Nanoparticle Formulations: A Study on the Effect of DNase-I on Microbial Biofilm and Wound Healing Activity. Mol Pharm. 2019;16:3916–25.
Harde H, Siddhapura K, Agrawal AK, Jain S. Divalent toxoids loaded stable chitosan-glucomannan nanoassemblies for efficient systemic, mucosal and cellular immunostimulatory response following oral administration. Elsevier B.V. Int J Pharm [Internet]. 2015;487:292–304. https://doi.org/10.1016/j.ijpharm.2015.04.042.
Harde H, Agrawal AK, Jain S. Trilateral, “3P” mechanics of stabilized layersomes technology for efficient oral immunization. J Biomed Nanotechnol. 2015;11:363–81.
Agrawal AK, Das M, Jain S. In situ gel systems as “smart” carriers for sustained ocular drug delivery. Expert Opin Drug Deliv. 2012;9:383–402.
Shenhar R, Rotello VM. Nanoparticles: Scaffolds and building blocks. Acc Chem Res. 2003;36:549–61.
Saha K, Bajaj A, Duncan B, Rotello VM. Beauty is skin deep: A surface monolayer perspective on nanoparticle interactions with cells and bio-macromolecules. Small. 2011;7:1903–18.
Kushwah V, Katiyar SS, Agrawal AK, Gupta RC, Jain S. Co-delivery of docetaxel and gemcitabine using PEGylated self-assembled stealth nanoparticles for improved breast cancer therapy. Elsevier Inc. Nanomed Nanotechnol: Biol Med [Internet]. 2018;14:1629–41. https://doi.org/10.1016/j.nano.2018.04.009.
Kushwah V, Katiyar SS, Dora CP, Kumar Agrawal A, Lamprou DA, Gupta RC, et al. Co-delivery of docetaxel and gemcitabine by anacardic acid modified self-assembled albumin nanoparticles for effective breast cancer management. Acta Biomater [Internet]. 2018;73:424–36. https://doi.org/10.1016/j.actbio.2018.03.057.
Singh S, Kushwah V, Agrawal AK, Jain S. Insulin- and quercetin-loaded liquid crystalline nanoparticles: Implications on oral bioavailability, antidiabetic and antioxidant efficacy. Nanomedicine. 2018;13:521–37.
Kushwah V, Agrawal AK, Dora CP, Mallinson D, Lamprou DA, Gupta RC, et al. Novel Gemcitabine Conjugated Albumin Nanoparticles: a Potential Strategy to Enhance Drug Efficacy in Pancreatic Cancer Treatment. Pharm Res. 2017;34:2295–311.
Albanese A, Sykes EA, Chan WCW. Rough around the edges: The inflammatory response of microglial cells to spiky nanoparticles. ACS Nano. 2010;4:2490–3.
Harde H, Siddhapura K, Agrawal AK, Jain S. Development of dual toxoid-loaded layersomes for complete immunostimulatory response following peroral administration. Nanomedicine. 2015;10:1077–91.
Jain S, Harde H, Indulkar A, Agrawal AK. Improved stability and immunological potential of tetanus toxoid containing surface engineered bilosomes following oral administration. Elsevier B.V. Nanomed Nanotechnol: Biol Med [Internet]. 2014;10:431–40. https://doi.org/10.1016/j.nano.2013.08.012.
Jain S, Kumar S, Agrawal AK, Thanki K, Banerjee UC. Enhanced transfection efficiency and reduced cytotoxicity of novel lipid-polymer hybrid nanoplexes. Mol Pharm. 2013;10:2416–25.
Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–8.
Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA. 2008;105:11613–8.
Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, et al. Renal clearance of nanoparticles. Nat Biotechnol [Internet]. 2007;25:1165–70. https://doi.org/10.1038/nbt1340.
Xie J, Lee S, Chen X. Nanoparticle-based theranostic agents. Adv Drug Deliv Rev. 2010;62:1064–79.
Doane TL, Burda C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem Soc Rev. 2012;41:2885–911.
Jain S, Garg T, Kushwah V, Thanki K, Agrawal AK, Dora CP. α-Tocopherol as functional excipient for resveratrol and coenzyme Q10-loaded SNEDDS for improved bioavailability and prophylaxis of breast cancer. Taylor & Francis. J Drug Target. 2017;25:554–65.
Aqil F, Kausar H, Agrawal AK, Jeyabalan J, Kyakulaga AH, Munagala R, et al. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Elsevier B.V. Exp Mol Pathol [Internet]. 2016;101:12–21. https://doi.org/10.1016/j.yexmp.2016.05.013.
Buttacavoli M, Albanese NN, Di Cara G, Alduina R, Faleri C, Gallo M, et al. Anticancer activity of biogenerated silver nanoparticles: An integrated proteomic investigation. Oncotarget. 2018;9:9685–705.
Zielinska E, Zauszkiewicz-Pawlak A, Wojcik M, Inkielewicz-Stepniak I. Silver nanoparticles of different sizes induce a mixed type of programmed cell death in human pancreatic ductal adenocarcinoma. Oncotarget. 2018;9:4675–97.
Yuan YG, Gurunathan S. Combination of graphene oxide-silver nanoparticle nanocomposites and cisplatin enhances apoptosis and autophagy in human cervical cancer cells. Int J Nanomedicine. 2017;12:6537–58.
Zhang XF, Gurunathan S. Combination of salinomycin and silver nanoparticles enhances apoptosis and autophagy in human ovarian cancer cells: An effective anticancer therapy. Int J Nanomedicine. 2016;11:3655–75.
Lin J, Huang Z, Wu H, Zhou W, Jin P, Wei P, et al. Inhibition of autophagy enhances the anticancer activity of silver nanoparticles. Autophagy. 2014;10:2006–20.
Liu P, Jin H, Guo Z, Ma J, Zhao J, Li D, et al. Silver nanoparticles outperform gold nanoparticles in radiosensitizing U251 cells in vitro and in an intracranial mouse model of glioma. Int J Nanomedicine [Internet]. 2016;11:5003–14. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5055115/pdf/ijn-11-5003.pdf.
Cordani M, Somoza Á. Targeting autophagy using metallic nanoparticles: a promising strategy for cancer treatment. Springer International Publishing. Cell Mol Life Sci [Internet]. 2019;76:1215–42. https://doi.org/10.1007/s00018-018-2973-y.
Ma X, Wu Y, Jin S, Tian Y, Zhang X, Zhao Y, et al. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano. 2011;5:8629–39.
Joshi P, Chakraborti S, Ramirez-Vick JE, Ansari ZA, Shanker V, Chakrabarti P, et al. The anticancer activity of chloroquine-gold nanoparticles against MCF-7 breast cancer cells. Elsevier B.V. Colloids Surf. B [Internet]. 2012;95:195–200. https://doi.org/10.1016/j.colsurfb.2012.02.039.
Crown J, O’Shaughnessy J, Gullo G. Emerging targeted therapies in triple-negative breast cancer. Elsevier Masson SAS. Ann Oncol [Internet]. 2012;23:vi56–65. https://doi.org/10.1093/annonc/mds196.
Zhang M, Kim HS, Jin T, Moon WK. Near-infrared photothermal therapy using EGFR-targeted gold nanoparticles increases autophagic cell death in breast cancer. Elsevier B.V. J Photochem Photobiol B Biol [Internet]. 2017;170:58–64. https://doi.org/10.1016/j.jphotobiol.2017.03.025.
Koken MHM, Smit EME, Jaspers-Dekker I, Oostra BA, Hagemeuer A, Bootsma D, et al. Localization of two human homologs, HHR6A and HHR6B, of the yeast DNA repair gene RAD6 to chromosomes Xq24-q25 and 5q23-q31. Genomics. 1992;12:447–53.
Koken MHM, Reynolds P, Jaspers-Dekker I, Prakash L, Prakash S, Bootsma D, et al. Structural and functional conservation of two human homologs of the yeast DNA repair gene RAD6. Proc Natl Acad Sci USA. 1991;88:8865–9.
Haynes B, Zhang Y, Liu F, Li J, Petit S, Kothayer H, et al. Gold nanoparticle conjugated Rad6 inhibitor induces cell death in triple negative breast cancer cells by inducing mitochondrial dysfunction and PARP-1 hyperactivation: Synthesis and characterization. Nanomedicine. 2016;12:745–57.
Ke S, Zhou T, Yang P, Wang Y, Zhang P, Chen K, et al. Gold nanoparticles enhance TRAIL sensitivity through Drp1-mediated apoptotic and autophagic mitochondrial fission in NSCLC cells. Int J Nanomedicine. 2017;12:2531–51.
Feng Q, Liu Y, Huang J, Chen K, Huang J, Xiao K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Springer US. Sci Rep [Internet]. 2018;8:1–13. https://doi.org/10.1038/s41598-018-19628-z.
Wang Y, Zi XY, Su J, Zhang HX, Zhang XR, Zhu HY, et al. Cuprous oxide nanoparticles selectively induce apoptosis of tumor cells. Int J Nanomedicine. 2012;7:2641–52.
Wang J, Gao S, Wang S, Xu Z, Wei L. Zinc oxide nanoparticles induce toxicity in CAL 27 oral cancer cell lines by activating PINK1/Parkin-mediated mitophagy. Int J Nanomedicine. 2018;13:3441–50.
Mozdoori N, Safarian S, Sheibani N. Augmentation of the cytotoxic effects of zinc oxide nanoparticles by MTCP conjugation: Non-canonical apoptosis and autophagy induction in human adenocarcinoma breast cancer cell lines. Mater Sci Eng C. 2017;78:949–59.
Khan MI, Mohammad A, Patil G, Naqvi SAH, Chauhan LKS, Ahmad I. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Elsevier Ltd. Biomaterials [Internet]. 2012;33:1477–88. https://doi.org/10.1016/j.biomaterials.2011.10.080.
Zhang X, Zhang H, Liang X, Zhang J, Tao W, Zhu X, et al. Iron oxide nanoparticles induce autophagosome accumulation through multiple mechanisms: Lysosome impairment, mitochondrial damage, and ER stress. Mol Pharm. 2016;13:2578–87.
Kuroda S, Tam J, Roth JA, Sokolov K, Ramesh R. EGFR-targeted plasmonic magnetic nanoparticles suppress lung tumor growth by abrogating G2/M cell-cycle arrest and inducing DNA damage. Int J Nanomedicine. 2014;9:3825–39.
Li X, Feng J, Zhang R, Wang J, Su T, Tian Z, et al. Quaternized chitosan/alginate-Fe3O4 magnetic nanoparticles enhance the chemosensitization of multidrug-resistant gastric carcinoma by regulating cell autophagy activity in mice. J Biomed Nanotechnol. 2016;12:948–61.
Wang Y, Yang F, Zhang HX, Zi XY, Pan XH, Chen F, et al. Cuprous oxide nanoparticles inhibit the growth and metastasis of melanoma by targeting mitochondria. Cell Death Dis. 2013;4:1–10.
Sun T, Yan Y, Zhao Y, Guo F, Jiang C. Copper oxide nanoparticles induce autophagic cell death in a549 cells. PLoS One. 2012;7:1–7.
Abudayyak M, Guzel EE, Özhan G. Copper (II) Oxide Nanoparticles Induced Nephrotoxicity In Vitro Conditions. Appl Vitr Toxicol. 2016;2:157–64.
Xia L, Wang Y, Chen Y, Yan J, Hao F, Su X, et al. Cuprous oxide nanoparticles inhibit the growth of cervical carcinoma by inducing autophagy. Oncotarget. 2017;8:61083–92.
Laha D, Pramanik A, Maity J, Mukherjee A, Pramanik P, Laskar A, et al. Interplay between autophagy and apoptosis mediated by copper oxide nanoparticles in human breast cancer cells MCF7. Elsevier B.V. Biochim Biophys Acta- Gen Subj [Internet]. 2014;1840:1–9. https://doi.org/10.1016/j.bbagen.2013.08.011.
Liu HL, Zhang YL, Yang N, Zhang YX, Liu XQ, Li CG, et al. A functionalized single-walled carbon nanotubeinduced autophagic cell death in human lung cells through Akt-TSC2-mTOR signaling. Cell Death Dis. 2011;2:1–7.
Wei P, Zhang L, Lu Y, Man N, Wen L. C60(Nd) nanoparticles enhance chemotherapeutic susceptibility of cancer cells by modulation of autophagy. Nanotechnology. 2010;21.
Ristic B, Harhaji-Trajkovic L, Bosnjak M, Dakic I, Mijatovic S, Trajkovic V. Modulation of cancer cell autophagic responses by graphene-based nanomaterials: Molecular mechanisms and therapeutic implications. Cancers (Basel). 2021;13:1–21.
Chen GY, Meng C Le, Lin KC, Tuan HY, Yang HJ, Chen CL, et al. Graphene oxide as a chemosensitizer: Diverted autophagic flux, enhanced nuclear import, elevated necrosis and improved antitumor effects. Elsevier Ltd. Biomaterials [Internet]. 2015;40:12–22. https://doi.org/10.1016/j.biomaterials.2014.11.034.
Lin KC, Lin MW, Hsu MN, Yu-Chen G, Chao YC, Tuan HY, et al. Graphene oxide sensitizes cancer cells to chemotherapeutics by inducing early autophagy events, promoting nuclear trafficking and necrosis. Theranostics. 2018;8:2477–87.
Arya BD, Mittal S, Joshi P, Pandey AK, Jaime E. Graphene oxide – chloroquine nanoconjugate induce necroptotic death in A549 cancer cells through autophagy modulation. Nanomedicine. 2018;13:2261–82.
Roggers R, Kanvinde S, Boonsith S, Oupický D. The practicality of mesoporous silica nanoparticles as drug delivery devices and progress toward this goal. AAPS PharmSciTech. 2014;15:1163–71.
Napierska D, Thomassen LCJ, Rabolli V, Lison D, Gonzalez L, Kirsch-Volders M, et al. Size-dependent cytotoxicity of monodisperse silica nanoparticles in human endothelial cells. Small. 2009;5:846–53.
Thomassen LCJ, Aerts A, Rabolli V, Lison D, Gonzalez L, Kirsch-Volders M, et al. Synthesis and characterization of stable monodisperse silica nanoparticle sols for in vitro cytotoxicity testing. Langmuir. 2010;26:328–35.
Krętowski R, Kusaczuk M, Naumowicz M, Kotyńska J, Szynaka B, Cechowska-Pasko M. The effects of silica nanoparticles on apoptosis and autophagy of glioblastoma cell lines. Nanomaterials. 2017;7:1–22.
Schütz I, Lopez-Hernandez T, Gao Q, Puchkov D, JaBerlinbs S, Nordmeyer D, et al. Lysosomal dysfunction caused by cellular accumulation of silica nanoparticles. J Biol Chem. 2016;291:14170–84.
Pool H, Campos-Vega R, Herrera-Hernández MG, García-Solis P, García-Gasca T, Sánchez IC, et al. Development of genistein-PEGylated silica hybrid nanomaterials with enhanced antioxidant and antiproliferative properties on HT29 human colon cancer cells. Am J Transl Res. 2018;10:2306–23.
Wang J, Yu Y, Lu K, Yang M, Li Y, Zhou X, et al. Silica nanoparticles induce autophagy dysfunction via lysosomal impairment and inhibition of autophagosome degradation in hepatocytes. Int J Nanomedicine. 2017;12:809–25.
Yu Y, Duan J, Yu Y, Li Y, Liu X, Zhou X, et al. Silica nanoparticles induce autophagy and autophagic cell death in HepG2 cells triggered by reactive oxygen species. Elsevier B.V. J Hazard Mater [Internet]. 2014;270:176–86. https://doi.org/10.1016/j.jhazmat.2014.01.028.
Wei F, Wang Y, Luo Z, Li Y, Duan Y. New findings of silica nanoparticles induced ER autophagy in human colon cancer cell. Sci Rep. 2017;7:1–11.
Patel KK, Tripathi M, Pandey N, Agrawal AK, Gade S, Anjum MM, et al. Alginate lyase immobilized chitosan nanoparticles of ciprofloxacin for the improved antimicrobial activity against the biofilm associated mucoid P. aeruginosa infection in cystic fibrosis. Elsevier. Int J Pharm [Internet]. 2019;563:30–42. https://doi.org/10.1016/j.ijpharm.2019.03.051.
Jain S, Kumar S, Agrawal AK, Thanki K, Banerjee UC. Hyaluronic acid-PEI-cyclodextrin polyplexes: Implications for in vitro and in vivo transfection efficiency and toxicity. RSC Adv. 2015;5:41144–54.
Harde H, Agrawal AK, Jain S. Tetanus toxoids loaded glucomannosylated chitosan based nanohoming vaccine adjuvant with improved oral stability and immunostimulatory response. Pharm Res. 2015;32:122–34.
Agrawal AK, Urimi D, Jain S. Multifunctional Polymeric Nano-Carriers. 2015.
Jain S, Spandana G, Agrawal AK, Kushwah V, Thanki K. Enhanced Antitumor Efficacy and Reduced Toxicity of Docetaxel Loaded Estradiol Functionalized Stealth Polymeric Nanoparticles. Mol Pharm. 2015;12:3871–84.
Eidi H, Joubert O, Némos C, Grandemange S, Mograbi B, Foliguet B, et al. Drug delivery by polymeric nanoparticles induces autophagy in macrophages. Elsevier B.V. Int J Pharm [Internet]. 2012;422:495–503. https://doi.org/10.1016/j.ijpharm.2011.11.020.
Li C, Liu H, Sun Y, Wang H, Guo F, Rao S, et al. PAMAM nanoparticles promote acute lung injury by inducing autophagic cell death through the Akt-TSC2-mTOR signaling pathway. J Mol Cell Biol. 2010;1:37–45.
Peynshaert K, Manshian BB, Joris F, Braeckmans K, De Smedt SC, Demeester J, et al. Exploiting intrinsic nanoparticle toxicity: The pros and cons of nanoparticle-induced autophagy in biomedical research. Chem Rev. 2014;114:7581–609.
Thomas TP, Majoros I, Kotlyar A, Mullen D, Holl MMB, Baker Jr JR. Cationic Poly(amidoamine) Dendrimer induces lysosomal apoptotic pathway at therapeutically relevant concentrations. Biomacromolecules [Internet]. 2009;10:3207–14. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3624763/pdf/nihms412728.pdf.
Saiyin W, Wang D, Li L, Zhu L, Liu B, Sheng L, et al. Sequential release of autophagy inhibitor and chemotherapeutic drug with polymeric delivery system for oral squamous cell carcinoma therapy. Mol Pharm. 2014;11:1662–75.
Armiñán A, Palomino-Schätzlein M, Deladriere C, Arroyo-Crespo JJ, Vicente-Ruiz S, Vicent MJ, et al. Metabolomics facilitates the discrimination of the specific anti-cancer effects of free- and polymer-conjugated doxorubicin in breast cancer models. Biomaterials. 2018;162:144–53.
Qiao ZY, Lai WJ, Lin YX, Li D, Nan XH, Wang Y, et al. Polymer-KLAK Peptide Conjugates Induce Cancer Cell Death through Synergistic Effects of Mitochondria Damage and Autophagy Blockage. Bioconjug Chem. 2017;28:1709–21.
Kushwah V, Jain DK, Agrawal AK, Jain S. Improved antitumor efficacy and reduced toxicity of docetaxel using anacardic acid functionalized stealth liposomes. Elsevier B.V. Colloids Surfaces B Biointerfaces [Internet]. 2018;172:213–23. https://doi.org/10.1016/j.colsurfb.2018.08.047.
Agrawal AK, Harde H, Thanki K, Jain S. Improved stability and antidiabetic potential of insulin containing folic acid functionalized polymer stabilized multilayered liposomes following oral administration. Biomacromol. 2014;15:350–60.
Jain S, Patil SR, Swarnakar NK, Agrawal AK. Oral delivery of doxorubicin using novel polyelectrolyte-stabilized liposomes (Layersomes). Mol Pharm. 2012;9:2626–35.
Man N, Chen Y, Zheng F, Zhou W, Wen LP. Induction of genuine autophagy by cationic lipids in mammalian cells. Autophagy. 2010;6:449–54.
Roberts R, Al-Jamal WT, Whelband M, Thomas P, Jefferson M, Van Den Bossche J, et al. Autophagy and formation of tubulovesicular autophagosomes provide a barrier against nonviral gene delivery. Autophagy. 2013;9:667–82.
Adiseshaiah PP, Clogston JD, McLeland CB, Rodriguez J, Potter TM, Neun BW, et al. Synergistic Combination Therapy with Nanoliposomal C6- Ceramide and Vinblastine is Associated with Autophagy Dysfunction in Hepatocarcinoma and Colorectal Cancer Models. Cancer Lett. 2013;337:254–65.
Condello M, Mancini G, Meschini S. The Exploitation of Liposomes in the Inhibition of Autophagy to Defeat Drug Resistance. Front Pharmacol. 2020;11:1–15.
Belfiore L, Saunders DN, Ranson M, Thurecht KJ, Storm G, Vine KL. Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: Challenges and opportunities. Elsevier B.V. J Control Release [Internet]. 2018;277:1–13. https://doi.org/10.1016/j.jconrel.2018.02.040.
Gilabert-Oriol R, Ryan GM, Leung AWY, Firmino NS, Bennewith KL, Bally MB. Liposomal formulations to modulate the tumour microenvironment and antitumour immune response. Int J Mol Sci 2018.
Lamichhane N, Udayakumar TS, D’Souza WD, Simone CB, Raghavan SR, Polf J, et al. Liposomes: Clinical applications and potential for image-guided drug delivery. Molecules. 2018;23:1–17.
Jeong JK, Gurunathan S, Kang MH, Han JW, Das J, Choi YJ, et al. Hypoxia-mediated autophagic flux inhibits silver nanoparticle-triggered apoptosis in human lung cancer cells. Nature Publishing Group. Sci Rep [Internet]. 2016;6:1–13. https://doi.org/10.1038/srep21688.
Lin J, Liu Y, Wu H, Huang Z, Ma J, Guo C, et al. Key Role of TFEB Nucleus Translocation for Silver Nanoparticle-Induced Cytoprotective Autophagy. Small. 2018;14:1–10.
Fageria L, Pareek V, Dilip RV, Bhargava A, Pasha SS, Laskar IR, et al. Biosynthesized Protein-Capped Silver Nanoparticles Induce ROS-Dependent Proapoptotic Signals and Prosurvival Autophagy in Cancer Cells. ACS Omega. 2017;2:1489–504.
Faedmaleki F, Shirazi FH, Salarian AA, Ashtiani HA, Rastegar H. Toxicity effect of silver nanoparticles on mice liver primary cell culture and HepG2 cell line. Iran J Pharm Res. 2014;13:235–42.
Chen Y, Yang T, Chen S, Qi S, Zhang Z, Xu Y. Silver nanoparticles regulate autophagy through lysosome injury and cell hypoxia in prostate cancer cells. J Biochem Mol Toxicol. 2020;34:1–9.
Al-kawmani AA, Alanazi KM, Farah MA, Ali MA, Hailan WAQ, Al-Hemaid FMA. Apoptosis-inducing potential of biosynthesized silver nanoparticles in breast cancer cells. J King Saud Univ Sci [Internet]. 2020;32:2480–8. https://doi.org/10.1016/j.jksus.2020.04.002.
Ren KW, Li YH, Wu G, Ren JZ, Lu H Bin, Li ZM, et al. Quercetin nanoparticles display antitumor activity via proliferation inhibition and apoptosis induction in liver cancer cells. Int J Oncol. 2017;50:1299–311.
Bhowmik T, Gomes A. NKCT1 (purified Naja kaouthia protein toxin) conjugated gold nanoparticles induced Akt/mTOR inactivation mediated autophagic and caspase 3 activated apoptotic cell death in leukemic cell. Elsevier Ltd. Toxicon [Internet]. 2016;121:86–97. https://doi.org/10.1016/j.toxicon.2016.08.004.
Kubota T, Kuroda S, Kanaya N, Morihiro T, Aoyama K, Kakiuchi Y, et al. HER2-targeted gold nanoparticles potentially overcome resistance to trastuzumab in gastric cancer. Elsevier Inc. Nanomed Nanotechnol: Biol Med [Internet]. 2018;14:1919–29. https://doi.org/10.1016/j.nano.2018.05.019.
Lou M, Zhang L na, Ji P gang, Feng F qiang, Liu J hui, Yang C, et al. Quercetin nanoparticles induced autophagy and apoptosis through AKT/ERK/Caspase-3 signaling pathway in human neuroglioma cells: In vitro and in vivo. Elsevier Masson SAS. Biomed Pharmacother [Internet]. 2016;84:1–9. https://doi.org/10.1016/j.biopha.2016.08.055.
Luo C lin, Liu Y qiong, Wang P, Song C hua, Wang K juan, Dai L ping, et al. The effect of quercetin nanoparticle on cervical cancer progression by inducing apoptosis, autophagy and anti-proliferation via JAK2 suppression. Elsevier Masson SAS. Biomed Pharmacother [Internet]. 2016;82:595–605. https://doi.org/10.1016/j.biopha.2016.05.029.
Ruan S, Xie R, Qin L, Yu M, Xiao W, Hu C, et al. Aggregable Nanoparticles-Enabled Chemotherapy and Autophagy Inhibition Combined with Anti-PD-L1 Antibody for Improved Glioma Treatment. Nano Lett. 2019;19:8318–32.
Bai DP, Zhang XF, Zhang GL, Huang YF, Gurunathan S. Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells. Int J Nanomedicine. 2017;12:6521–35.
Farasat M, Niazvand F, Khorsandi L. Zinc oxide nanoparticles induce necroptosis and inhibit autophagy in MCF-7 human breast cancer cells. Biologia (Bratisl). 2020;75:161–74.
Ren X, Chen Y, Peng H, Fang X, Zhang X, Chen Q, et al. Blocking Autophagic Flux Enhances Iron Oxide Nanoparticle Photothermal Therapeutic Efficiency in Cancer Treatment. ACS Appl Mater Interfaces. 2018;10:27701–11.
Huang D, Zhou H, Gao J. Nanoparticles modulate autophagic effect in a dispersity-dependent manner. Nature Publishing Group. Sci Rep. 2015;5:1–10.
Xie Y, Jiang J, Tang Q, Zou H, Zhao X, Liu H, et al. Iron Oxide Nanoparticles as Autophagy Intervention Agents Suppress Hepatoma Growth by Enhancing Tumoricidal Autophagy. Adv Sci. 2020;1903323:1–13.
Lin YR, Chan CH, Lee HT, Cheng SJ, Yang JW, Chang SJ, et al. Remote magnetic control of autophagy in mouse b-lymphoma cells with iron oxide nanoparticles. Nanomaterials. 2019;9:1–13.
Du S, Li J, Du C, Huang Z, Chen G, Yan W. Overendocytosis of superparamagnetic iron oxide particles increases apoptosis and triggers autophagic cell death in human osteosarcoma cell under a spinning magnetic field. Oncotarget. 2017;8:9410–24.
Levada K, Pshenichnikov S, Omelyanchik A, Rodionova V, Nikitin A, Savchenko A, et al. Progressive lysosomal membrane permeabilization induced by iron oxide nanoparticles drives hepatic cell autophagy and apoptosis. Springer Singapore. Nano Converg [Internet]. 2020;7:1–17. https://doi.org/10.1186/s40580-020-00228-5.
Shi M, Cheng L, Zhang Z, Liu Z, Mao X. Ferroferric oxide nanoparticles induce prosurvival autophagy in human blood cells by modulating the Beclin 1/Bcl-2/VPs34 complex. Int J Nanomedicine. 2015;10:207–16.
Jiang YW, Gao G, Jia HR, Zhang X, Zhao J, Ma N, et al. Copper oxide nanoparticles induce enhanced radiosensitizing effect via destructive autophagy. American Chemical Society. ACS Biomater Sci Eng. 2019;5:1569–79.
Xiong Q, Liu A, Ren Q, Xue Y, Yu X, Ying Y, et al. Cuprous oxide nanoparticles trigger reactive oxygen species-induced apoptosis through activation of erk-dependent autophagy in bladder cancer. Springer US. Cell Death Dis [Internet]. 2020;11:1–13. https://doi.org/10.1038/s41419-020-2554-5.
Li X, Xu H, Li C, Qiao G, Farooqi AA, Gedanken A, et al. Zinc-doped copper oxide nanocomposites inhibit the growth of pancreatic cancer by inducing autophagy through AMPK/mTOR pathway. Front Pharmacol. 2019;10:1–11.
Nowak JS, Mehn D, Nativo P, García CP, Gioria S, Ojea-Jiménez I, et al. Silica nanoparticle uptake induces survival mechanism in A549 cells by the activation of autophagy but not apoptosis. Toxicol Lett. 2014;224:84–92.
Li Y, Cho MH, Lee SS, Lee DE, Cheong H, Choi Y. Hydroxychloroquine-loaded hollow mesoporous silica nanoparticles for enhanced autophagy inhibition and radiation therapy. Elsevier B.V. J Control Release [Internet]. 2020;325:100–10. https://doi.org/10.1016/j.jconrel.2020.06.025.
Chen T, Cen D, Ren Z, Wang Y, Cai X, Huang J, et al. Bismuth embedded silica nanoparticles loaded with autophagy suppressant to promote photothermal therapy. Elsevier. Biomaterials [Internet]. 2019;221:1–9. https://doi.org/10.1016/j.biomaterials.2019.119419.
Zhang X, Dong Y, Zeng X, Liang X, Li X, Tao W, et al. The effect of autophagy inhibitors on drug delivery using biodegradable polymer nanoparticles in cancer treatment. Elsevier Ltd. Biomaterials [Internet]. 2014;35:1932–43. https://doi.org/10.1016/j.biomaterials.2013.10.034.
Zhang X, Yang Y, Liang X, Zeng X, Liu Z, Tao W, et al. Enhancing therapeutic effects of docetaxel-loaded dendritic copolymer nanoparticles by co-treatment with autophagy inhibitor on breast cancer. Theranostics. 2014;4:1085–95.
Wang FZ, Xing L, Tang ZH, Lu JJ, Cui PF, Qiao JB, et al. Codelivery of Doxorubicin and shAkt1 by Poly(ethylenimine)-Glycyrrhetinic Acid Nanoparticles to Induce Autophagy-Mediated Liver Cancer Combination Therapy. Mol Pharm. 2016;13:1298–307.
Feng Y, Gao Y, Wang D, Xu Z, Sun W, Ren P. Autophagy Inhibitor (LY294002) and 5-fluorouracil (5-FU) Combination-Based Nanoliposome for Enhanced Efficacy Against Esophageal Squamous Cell Carcinoma. Nanoscale Res Lett. 2018;13:1–9.
Zhang J, Zhu S, Tan Q, Cheng D, Dai Q, Yang Z, et al. Combination therapy with ropivacaine-loaded liposomes and nutrient deprivation for simultaneous cancer therapy and cancer pain relief. Theranostics. 2020;10:4885–99.
Chang CM, Lan KL, Huang WS, Lee YJ, Lee TW, Chang CH, et al. 188Re-liposome can induce mitochondrial autophagy and reverse drug resistance for ovarian cancer: From bench evidence to preliminary clinical proof-of-concept. Int J Mol Sci. 2017;18:1–15.
Jiang GM, Tan Y, Wang H, Peng L, Chen HT, Meng XJ, et al. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol Cancer. 2019;18:1–22.
Acknowledgements
Authors are grateful to the Indian Institute of Technology (Banaras Hindu University), Varanasi, for providing infrastructure facilities. Dulla Naveen Kumar is thankful to SERB for providing financial assistance in terms of JRF in one of the SERB sponsored projects (SRG/2019/000150).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Shloka Negi was involved in literature search and writing of article; Aiswarya Chaudhuri sketched images and structured the article; Dulla Naveen Kumar formatted the tables; Deepa Dehari compiled the data; Sanjay Singh corrected and restructured the article; Ashish Kumar Agrawal took part in literature search, refinement, and improvement of article.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interests
The authors declare that they have no conflicts of interest with the contents of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Negi, S., Chaudhuri, A., Kumar, D.N. et al. Nanotherapeutics in autophagy: a paradigm shift in cancer treatment. Drug Deliv. and Transl. Res. 12, 2589–2612 (2022). https://doi.org/10.1007/s13346-022-01125-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01125-6