Abstract
Achieving efficacious systemic levels of orally administered peptides is incredibly challenging due to the significant barriers to their bioavailability—their stability in the gastrointestinal tract and challenge of transepithelial transit, and variable pharmacokinetics. Even so, as the generally preferred route of administration, significant research effort in academic and industrial settings has focused on enabling the systemic absorption of orally delivered peptides. Despite several decades of research, few have ever reached the market. The recent approval of Rybelsus® (oral semaglutide) by the FDA [1], the EMA [2], and the Pmda [3] represents a significant landmark in the delivery of therapeutic peptides and is the culmination of more than 30 years research and development of the drug delivery technology enabling the product—Emisphere’s Eligen™ technology—and an outstanding commitment to scientific, technical, and clinical innovation by Novo Nordisk. Following years of fundamental and applied research, an innovative clinical strategy led to the aptly named PIONEER clinical programme. This included ten Phase 3 clinical trials that demonstrated the tablet formulation to be as effective as the already approved injectable form of the drug, and more effective than competitor products in terms of its blood glucose lowering effects and weight loss. Not only is this a potentially life changing medicine for diabetic patients, it holds tremendous commercial potential for Novo Nordisk, with some analysts predicting the product to reach $5 billion in peak revenues [3]. In this “Inspirational Note,” we summarize some of the public domain work that led to the achievement of this significant milestone and provide commentary on its potential future impact.

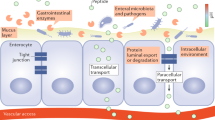
Reproduced with permission from Baekdal et al. [18]

Reproduced with permission from Baekdal et al. [18]

Adapted from the Novo Nordisk Capital Markets Day 2017 [25]
Similar content being viewed by others
References
https://www.novonordisk.com/content/dam/Denmark/HQ/investors/irmaterial/investor_presentations/2019/20190923%20-%20Rybelsus%20presentation.pdf, Accessed on 10th May 2021.
https://www.novonordisk.com/content/dam/Denmark/HQ/investors/irmaterial/investor_presentations/2020/05052020_Q1%202020%20core%20deck.pdf, Accessed 10th May 2021.
https://www.pmlive.com/pharma_news/novo_nordisk_gets_another_ok_for_oral_glp-1_drug_rybelsus_1343323, Accessed 10th May 2021.
Lewis AL, Richard J. Ther Deliv. 2015;6(2):149–63.
Anselmo AC, Gokarn Y, Mitragotri S. Nat Rev Drug Discov. 2019;18:19–39.
Maher S, Mrsny RJ, Brayden DJ. Adv Drug Deliv Rev. 2016;106(Pt B):277–319.
Aguirre TA, Teijeiro-Osorio D, Rosa M, Coulter IS, Alonso MJ, Brayden DJ. Adv Drug Deliv Rev. 2016;106(Pt B):277–319.
Drucker DJ. Nat Rev Drug Discov. 2020;19:277–89.
https://www.novonordisk.com/content/dam/Denmark/HQ/investors/irmaterial/annual_report/2009/20090130_Annual%20Report%202008_UK.pdf, Accessed 18th April 2020.
Abramson A, et al. Science. 2019;363(6427):611–5.
https://emisphere.com/partnerships/, Accessed 4th August 2020.
https://www.pharmaceuticalonline.com/doc/merrion-announces-license-agreement-with-novo-0001, Accessed 4th August 2020.
https://www.biospace.com/article/merrion-pharma-looks-to-wind-up-operations-announces-liquidation-plans-/, Accessed 4th August 2020.
Presented at Experimental Biology Conference 2011, Abstract number 9314, Poster Number LB394.
https://ir.emisphere.com/news-releases/news-release-details/emisphere-technologies-inc-announces-financial-results-fourth, Accessed 4th August 2020.
https://ir.emisphecom/news-releases/news-release-details/emisphere-launches-eligen-b12tm-first-oral-prescription-tablet, Accessed 4th August 2020.
Presented at the American Diabetes Association, 77th Annual Scientific Sessions, 2017 San Diego CA USA.
Baekdal et al. Clinical Pharmacology in Drug Development 2021:1–10.
Buckley et al. Sci Transl Med 2018;10:eaar7047.
FDA Drug Approval Package for Rybelsus. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/213051Orig1s000TOC.cfm, Accessed 13th May 2021.
FDA Drug Approval Package for Ozempic. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209637Orig1s000TOC.cfm, Accessed 13th May 2021.
https://www.outsourcing-pharma.com/Article/2015/08/27/Novo-Nordisk-to-pump-2bn-into-network-on-back-of-oral-GLP-1-milestone, Accessed 13th May 2021.
https://diabetes.medicinematters.com/en-GB/semaglutide/cardiovascular-outcomes/a-quick-guide-to-the-pioneer-trials/16877792, Accessed 13th May 2021.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209637s003lbl.pdf, Accessed 13th May 2021.
https://www.novonordisk.com/content/dam/nncorp/global/en/investors/irmaterial/cmd/2017/00_CMD%20Presentation%20combined.pdf, Accessed 13th May 2021.
https://pharmaphorum.com/news/novos-new-oral-glp-1-still-has-obstacles-to-overcome/, Accessed 13th May 2021.
https://www.fiercepharma.com/marketing/novo-prices-oral-rybelsus-par-injectables-ending-investor-discounting-fears, Accessed 13th May 2021.
Kong et al. Nat Biomed Eng. 2020;4:560–571.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lewis, A.L., McEntee, N., Holland, J. et al. Development and approval of rybelsus (oral semaglutide): ushering in a new era in peptide delivery. Drug Deliv. and Transl. Res. 12, 1–6 (2022). https://doi.org/10.1007/s13346-021-01000-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-01000-w