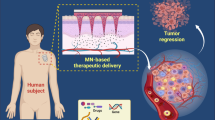
Abstract
Regarding the increasing prevalence of cancer throughout the globe, the development of novel alternatives for conventional therapies is inevitable to circumvent limitations such as low efficacy, complications, and high cost. Recently, microneedle arrays (MNs) have been introduced as a novel, minimally invasive, and low-cost approach. MNs can delivery both small molecule and macromolecular drugs or even nanoparticles (NPs) to the tumor tissue in a safe and controlled manner. Relying on the recent promising outcomes of MNs in transdermal delivery of anticancer agents, this review is aimed to summarize constituent materials, fabrication methods, advantages, and limitations of different types of MNs used in cancer therapy applications. This review paper also presents the potential use of MNs in transdermal delivery of NPs for effective chemotherapy, gene therapy, immunotherapy, photodynamic, and photothermal therapy. Additionally, MNs are currently explored as routine point-of-care health monitoring devices for transdermal detection of cancer biomarkers or physiologically relevant analytes which will be addressed in this paper. Despite the promising potential of MNs for cancer therapy and diagnosis, several limitations have impeded their therapeutic efficacy and real-time applicability that are addressed in this paper.









Similar content being viewed by others
Abbreviations
- 1-MT:
-
1-Methyl-DL-tryptophan
- mHA:
-
1-MT-conjugated HA
- 5-ALA:
-
5-Aminolevulinic acid
- 5-FU:
-
5-Fluorouracil
- Ac-DEX:
-
Acetal-modified dextran
- aCTLA4:
-
Anti-CTLA4
- APC:
-
Antigen-presenting cells
- aPD1:
-
Anti-PD-1
- AuNCs:
-
Au nanocages
- CNTs:
-
Carbon nanotubes
- CMC:
-
Carboxymethyl cellulose
- CEA:
-
Carcinoembryonic antigen
- CSMNs:
-
Core-shell structure microneedles
- CLIP:
-
Continuous liquid interface production
- PMVE/MA:
-
Copolymer of methyl vinyl ether and maleic anhydride
- CMOS:
-
Complementary metal-oxide-semiconductor
- CTL:
-
Cytolytic T lymphocytes
- CTLA4:
-
Cytotoxic T lymphocyte-associated molecule-4
- DAMPs:
-
Damage-associated molecular patterns
- DCs:
-
Dendritic cells
- DTX:
-
Docetaxel
- DOX:
-
Doxorubicin
- Teffs:
-
Effective T cells
- GOx:
-
Glucose oxidase
- GFNs:
-
Graphene family nanomaterials
- HPV:
-
Human papillomavirus
- HA:
-
Hyaluronic acid
- HAase:
-
Hyaluronidase
- HEM:
-
Hybrid electro-MNs
- ICG:
-
Indocyanine green
- iNOS:
-
Inducible NO synthase
- IP:
-
Intraperitoneal
- I.V.:
-
Intravenous
- LCs:
-
Langerhans cells
- LaB6:
-
Lanthanum hexaboride
- LDH:
-
Layered double hydroxides
- LCC-NPs:
-
Lipid-coated cisplatin nanoparticles
- LIGA:
-
Lithography, electroplating, and molding
- CPP-PEI1800-Man:
-
Mannosylated TAT peptide-grafted low molecular weight PEI
- MBGs:
-
Mesoporous bioactive glasses
- MSNs:
-
Mesoporous silica nanoparticles
- MPEG-PDLLA-DTX:
-
Methoxy-poly (ethylene glycol) poly (D, L-lactide) micelles
- ZnONW-MGP:
-
Microbubble generator probe
- MIM:
-
Microinjection molding
- MNs:
-
Microneedle arrays
- MDR:
-
Multi-drug resistance
- NCs:
-
Nanocarriers
- NPs:
-
Nanoparticles
- NLCs:
-
Nanostructured lipid carriers
- NIR:
-
Near infrared
- NO:
-
Nitric oxide
- R8:
-
Octaarginine
- OSM-(PEG-PAEU):
-
Oligo sulfamethazine conjugated poly (β-amino ester urethane)
- OCT:
-
Optical coherence tomography
- OVA:
-
Ovalbumin
- PTX:
-
Paclitaxel
- GNR-PEG:
-
PEGylated gold nanorods
- PDT:
-
Photodynamic therapy
- PTT:
-
Photothermal therapy
- PC:
-
Phthalocyanine
- PVD:
-
Physical vapor deposition
- POC:
-
Point-of-care
- PEDOT:
-
Poly (3, 4-ethylenedioxythiophene)
- PLGA:
-
Poly D,L-lactic-co-glycolic acid
- PLLA:
-
Poly L-lactic acid
- PMVE/MA-PE:
-
Poly methyl vinyl ether-co-maleic acid pectin
- PHEMA:
-
Poly 2-hydroxyethyl methacrylate
- PMVE/MA-PEG:
-
Poly methyl vinyl ether/maleic acid-poly ethylene glycol
- PCL:
-
Polycaprolactone
- PC:
-
Polycarbonate
- PD:
-
Polydopamine
- PEGDA:
-
Polyethylene glycol diacrylate
- PEI:
-
Polyethylenimine
- POM:
-
Polyformaldehyde
- γ-PGA:
-
Polyglutamic acid
- PGA:
-
Polyglycolic acid
- PLA:
-
Polylactic acid
- PMMA:
-
Polymethyl methacrylate
- PS-b-PAA:
-
Polystyrene-block-poly acrylic acid
- PVA:
-
Polyvinyl alcohol
- PDL1:
-
Programmed cell death ligand-1
- PD1:
-
Programmed cell death receptor-1
- PSCA:
-
Prostate stem cell antigen
- PPIX:
-
Protoporphyrin IX
- PB:
-
Prussian blue
- p53 DNA:
-
p53 tumor suppressor gene
- RIE:
-
Reactive ion etching
- RALA:
-
Arginine-alanine-leucine-alanine
- Treg:
-
Regulatory T cells
- R848:
-
Resiquimod
- pRb:
-
Retinoblastoma protein
- SEM:
-
Scanning electron microscopy
- STAT3:
-
Signal transducer and activity of transcription 3
- siRNA:
-
Small interfering RNA
- NaCMC:
-
Sodium carboxymethyl cellulose
- SLNs:
-
Solid lipid nanoparticles
- SC:
-
Stratum corneum
- TT:
-
Tetanus toxoid
- TDD:
-
Transdermal delivery
- TAAs:
-
Tumor-associated antigens
- tdLN:
-
Tumor-draining lymph node
- TYR:
-
Tyrosinase
- TRP-2:
-
Tyrosinase-related protein-2
- US:
-
Ultrasound
- VEGF:
-
Vascular endothelial growth factor
- VA-CNT/PI:
-
Vertically aligned carbon nanotubes/polyimide
- ZnONWs:
-
Zinc oxide nanowires
- ZnPc:
-
Zinc phthalocyanine
References
Atlihan-Gudogdu E, Ilem-Ozdemir D, Ekinci M, Ozgenc E, Demir ES, Sánchez-Dengra B, et al. Recent developments in cancer therapy and diagnosis. J Pharm Investig. 2020. https://doi.org/10.1007/s40005-020-00473-0.
Carter P, Narasimhan B, Wang Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int J Pharm. 2019;555:49–62. https://doi.org/10.1016/j.ijpharm.2018.11.032.
Abolmaali SS, Tamaddon AM, Dinarvand R. A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer Chemother Pharmacol. 2013;71(5):1115–30.
Javanmardi S, Reza Aghamaali M, Sadat Abolmaali S, Mohammadi S, Mohammad Tamaddon A. miR-21, an oncogenic target miRNA for cancer therapy: molecular mechanisms and recent advancements in chemo and radio-resistance. Curr Gene Ther. 2016;16(6):375–89.
Peres J, Kwesi-Maliepaard EM, Rambow F, Larue L, Prince S. The tumour suppressor, miR-137, inhibits malignant melanoma migration by targetting the TBX3 transcription factor. Cancer Lett. 2017;405:111–9. https://doi.org/10.1016/j.canlet.2017.07.018.
Zhang W, Song Y, Eldi P, Guo X, Hayball JD, Garg S, et al. Targeting prostate cancer cells with hybrid elastin-like polypeptide/liposome nanoparticles. Int J Nanomed. 2018;13:293.
Qiu Y, Arcadia C, Alibakhshi MA, Rosenstein J, Wanunu M. Nanopore fabrication in ultrathin HFO2 membranes for nanopore-based DNA sequencing. BPJ. 2018;114(3):179a.
Taghizadeh S, Alimardani V, Roudbali PL, Ghasemi Y, Kaviani E. Gold nanoparticles application in liver cancer. Photodiagn Photodyn Ther. 2019;25:389–400. https://doi.org/10.1016/j.pdpdt.2019.01.027.
Alexander A, Dwivedi S, Giri TK, Saraf S, Saraf S, Tripathi DK. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J Control Release. 2012;164(1):26–40.
Pegoraro C, MacNeil S, Battaglia G. Transdermal drug delivery: from micro to nano. Nanoscale. 2012;4(6):1881–94.
Das Kurmi B, Tekchandani P, Paliwal R, Rai Paliwal S. Transdermal drug delivery: opportunities and challenges for controlled delivery of therapeutic agents using nanocarriers. Curr Drug Metab. 2017;18(5):481–95.
Yin Q, Shen J, Zhang Z, Yu H, Li Y. Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Adv Drug Del Rev. 2013;65(13–14):1699–715.
Lee H, Song C, Baik S, Kim D, Hyeon T, Kim D-H. Device-assisted transdermal drug delivery. Adv Drug Del Rev. 2018;127:35–45. https://doi.org/10.1016/j.addr.2017.08.009.
Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261.
Indermun S, Luttge R, Choonara YE, Kumar P, Du Toit LC, Modi G, et al. Current advances in the fabrication of microneedles for transdermal delivery. J Control Release. 2014;185:130–8.
Leone M, Mönkäre J, Bouwstra J, Kersten G. Dissolving microneedle patches for dermal vaccination. Pharm Res. 2017;34(11):2223–40.
Bhatnagar S, Gadeela PR, Thathireddy P, Venuganti VVK. Microneedle-based drug delivery: materials of construction. J Chem Sci. 2019;131(9):90.
Mönkäre J, Pontier M, van Kampen EE, Du G, Leone M, Romeijn S, et al. Development of PLGA nanoparticle loaded dissolving microneedles and comparison with hollow microneedles in intradermal vaccine delivery. Eur J Pharm Biopharm. 2018;129:111–121.
Cole G, Ali AA, McCrudden CM, McBride JW, McCaffrey J, Robson T, et al. DNA vaccination for cervical cancer: strategic optimisation of RALA mediated gene delivery from a biodegradable microneedle system. Eur J Pharm Biopharm. 2018;127:288–97.
Tasca F, Tortolini C, Bollella P, Antiochia R. Microneedle-based electrochemical devices for transdermal biosensing: a review. Curr Opin Electrochem. 2019;16:42–9. https://doi.org/10.1016/j.coelec.2019.04.003.
Miller PR, Skoog SA, Edwards TL, Lopez DM, Wheeler DR, Arango DC, et al. Multiplexed microneedle-based biosensor array for characterization of metabolic acidosis. Talanta. 2012;88:739–42.
Prausnitz MR. Engineering microneedle patches for vaccination and drug delivery to skin. Ann Rev Chem Biomol Eng. 2017;8:177–200.
Henry S, McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles: a novel approach to transdermal drug delivery. J Pharm Sci. 1998;87(8):922–5.
Dharadhar S, Majumdar A, Dhoble S, Patravale V. Microneedles for transdermal drug delivery: a systematic review. Drug Dev Ind Pharm. 2019;45(2):188–201.
Qiu Y, Gao Y, Hu K, Li F. Enhancement of skin permeation of docetaxel: a novel approach combining microneedle and elastic liposomes. J Control Release. 2008;129(2):144–50.
Naguib YW, Kumar A, Cui Z. The effect of microneedles on the skin permeability and antitumor activity of topical 5-fluorouracil. Acta Pharm Sin B. 2014;4(1):94–9. https://doi.org/10.1016/j.apsb.2013.12.013.
Chablani L, Tawde SA, Akalkotkar A, D’Souza MJ. Evaluation of a particulate breast cancer vaccine delivered via skin. AAPS J. 2019;21(2):12.
Zandi A, Khayamian MA, Saghafi M, Shalileh S, Katebi P, Assadi S, et al. Microneedle-based generation of microbubbles in cancer tumors to improve ultrasound-assisted drug delivery. Adv Healthc Mater. 2019;8(17):1900613. https://doi.org/10.1002/adhm.201900613.
Ahmed KS, Shan X, Mao J, Qiu L, Chen J. Derma roller® microneedles-mediated transdermal delivery of doxorubicin and celecoxib co-loaded liposomes for enhancing the anticancer effect. Mater Sci Eng C. 2019;99:1448–58.
Tham HP, Xu K, Lim WQ, Chen H, Zheng M, Thng TGS, et al. Microneedle-assisted topical delivery of Photodynamically active mesoporous formulation for combination therapy of deep-seated melanoma. ACS Nano. 2018;12(12):11936–48. https://doi.org/10.1021/acsnano.8b03007.
Hu Y, Xu B, Xu J, Shou D, Liu E, Gao J, et al. Microneedle-assisted dendritic cell-targeted nanoparticles for transcutaneous DNA immunization. Polym Chem. 2015;6(3):373–9.
Keum DH, Jung HS, Wang T, Shin MH, Kim YE, Kim KH, et al. Microneedle biosensor for real-time electrical detection of nitric oxide for in situ cancer diagnosis during Endomicroscopy. Adv Healthc Mater. 2015;4(8):1153–8. https://doi.org/10.1002/adhm.201500012.
Kim CS, Wilder-Smith PB, Ahn Y-C, Liaw L-HL, Chen Z, Kwon YJ. Enhanced detection of early-stage oral cancer in vivo by optical coherence tomography using multimodal delivery of gold nanoparticles. JBO. 2009;14(3):034008.
Chen L, Zhang C, Xiao J, You J, Zhang W, Liu Y, et al. Local extraction and detection of early stage breast cancers through a microneedle and nano-ag/MBL film based painless and blood-free strategy. Mater Sci Eng C. 2020;109:110402.
Song S, Na J, Jang M, Lee H, Lee H-S, Lim Y-B et al. A CMOS VEGF sensor for cancer diagnosis using a peptide aptamer-based functionalized microneedle. IEEE transactions on biomedical circuits and systems. 2019;13(6):1288–1299.
Uddin MJ, Scoutaris N, Klepetsanis P, Chowdhry B, Prausnitz MR, Douroumis D. Inkjet printing of transdermal microneedles for the delivery of anticancer agents. Int J Pharm. 2015;494(2):593–602.
Ma Y, Boese SE, Luo Z, Nitin N, Gill HS. Drug coated microneedles for minimally-invasive treatment of oral carcinomas: development and in vitro evaluation. BioMi. 2015;17(2):44. https://doi.org/10.1007/s10544-015-9944-y.
Ruan W, Zhai Y, Yu K, Wu C, Xu Y. Coated microneedles mediated intradermal delivery of octaarginine/BRAF siRNA nanocomplexes for anti-melanoma treatment. Int J Pharm. 2018;553(1):298–309. https://doi.org/10.1016/j.ijpharm.2018.10.043.
Jain AK, Lee CH, Gill HS. 5-Aminolevulinic acid coated microneedles for photodynamic therapy of skin tumors. J Control Release. 2016;239:72–81.
Duong HTT, Yin Y, Thambi T, Nguyen TL, Giang Phan VH, Lee MS, et al. Smart vaccine delivery based on microneedle arrays decorated with ultra-pH-responsive copolymers for cancer immunotherapy. Biomaterials. 2018;185:13–24. https://doi.org/10.1016/j.biomaterials.2018.09.008.
Mansoor I, Lai J, Ranamukhaarachchi S, Schmitt V, Lambert D, Dutz J, et al. A microneedle-based method for the characterization of diffusion in skin tissue using doxorubicin as a model drug. BioMi. 2015;17(3):61.
Jung YS, Koo D-H, Yang J-Y, Lee H-Y, Park J-H, Park JH. Peri-tumor administration of 5-fluorouracil sol-gel using a hollow microneedle for treatment of gastric cancer. Drug Deliv. 2018;25(1):872–9.
Tang T, Deng Y, Chen J, Zhao Y, Yue R, Choy KW, et al. Local administration of siRNA through microneedle: optimization, bio-distribution, tumor suppression and toxicity. Sci Rep. 2016;6(1):30430. https://doi.org/10.1038/srep30430.
Tang T, Deng Y, Chen J, Zhao Y, Yue R, Choy KW, et al. Local administration of siRNA through microneedle: optimization, bio-distribution, tumor suppression and toxicity. Sci Rep. 2016;6(1):1–8.
Nguyen HX, Bozorg BD, Kim Y, Wieber A, Birk G, Lubda D, et al. Poly (vinyl alcohol) microneedles: fabrication, characterization, and application for transdermal drug delivery of doxorubicin. Eur J Pharm Biopharm. 2018;129:88–103.
Ingrole RS, Gill HS. Microneedle coating methods: a review with a perspective. J Pharmacol Exp Ther. 2019;370(3):555–69.
Lan X, She J, Lin D-a, Xu Y, Li X, Yang W-f, et al. Microneedle-mediated delivery of lipid-coated cisplatin nanoparticles for efficient and safe Cancer therapy. ACS Appl Mater Interfaces. 2018;10(39):33060–9.
Bhatnagar S, Bankar NG, Kulkarni MV, Venuganti VVK. Dissolvable microneedle patch containing doxorubicin and docetaxel is effective in 4T1 xenografted breast cancer mouse model. Int J Pharm. 2019;556:263–75. https://doi.org/10.1016/j.ijpharm.2018.12.022.
Wang PM, Cornwell M, Hill J, Prausnitz MR. Precise microinjection into skin using hollow microneedles. J Invest Dermatol. 2006;126(5):1080–7. https://doi.org/10.1038/sj.jid.5700150.
Zhou Z, Pang J, Wu X, Wu W, Chen X, Kong M. Reverse immune suppressive microenvironment in tumor draining lymph nodes to enhance anti-PD1 immunotherapy via nanovaccine complexed microneedle. Nano Res. 2020:1–10. https://doi.org/10.1007/s12274-020-2737-5.
Chen M-C, Lin Z-W, Ling M-H. Near-infrared light-activatable microneedle system for treating superficial tumors by combination of chemotherapy and Photothermal therapy. ACS Nano. 2016;10(1):93–101. https://doi.org/10.1021/acsnano.5b05043.
Hao Y, Dong M, Zhang T, Peng J, Jia Y, Cao Y, et al. Novel approach of using near-infrared responsive PEGylated gold Nanorod coated poly(l-lactide) microneedles to enhance the antitumor efficiency of docetaxel-loaded MPEG-PDLLA micelles for treating an A431 tumor. ACS Appl Mater Interfaces. 2017;9(18):15317–27. https://doi.org/10.1021/acsami.7b03604.
Moreira AF, Rodrigues CF, Jacinto TA, Miguel SP, Costa EC, Correia IJ. Poly (vinyl alcohol)/chitosan layer-by-layer microneedles for cancer chemo-photothermal therapy. Int J Pharm. 2020;576:118907.
Pei P, Yang F, Liu J, Hu H, Du X, Hanagata N, et al. Composite-dissolving microneedle patches for chemotherapy and photothermal therapy in superficial tumor treatment. Biomater Sci. 2018;6(6):1414–23.
Hao Y, Chen Y, He X, Yang F, Han R, Yang C, et al. Near-infrared responsive 5-fluorouracil and indocyanine green loaded MPEG-PCL nanoparticle integrated with dissolvable microneedle for skin cancer therapy. Bioactive Mater. 2020;5(3):542–52. https://doi.org/10.1016/j.bioactmat.2020.04.002.
Chen M, Quan G, Wen T, Yang P, Qin W, Mai H, et al. Cold to hot: binary cooperative microneedle array-amplified Photoimmunotherapy for eliciting antitumor immunity and the abscopal effect. ACS Appl Mater Interfaces. 2020. https://doi.org/10.1021/acsami.0c05090.
Zhao X, Li X, Zhang P, Du J, Wang Y. Tip-loaded fast-dissolving microneedle patches for photodynamic therapy of subcutaneous tumor. J Control Release. 2018;286:201–9.
Chen S-X, Ma M, Xue F, Shen S, Chen Q, Kuang Y, et al. Construction of microneedle-assisted co-delivery platform and its combining photodynamic/immunotherapy. J Control Release. 2020;324:218–27. https://doi.org/10.1016/j.jconrel.2020.05.006.
Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016;16(4):2334–40. https://doi.org/10.1021/acs.nanolett.5b05030.
Ye Y, Wang J, Hu Q, Hochu GM, Xin H, Wang C, et al. Synergistic transcutaneous immunotherapy enhances antitumor immune responses through delivery of checkpoint inhibitors. ACS Nano. 2016;10(9):8956–63.
Steinman RM. Lasker Basic Medical Research Award. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13:1155.
Ye Y, Wang C, Zhang X, Hu Q, Zhang Y, Liu Q, et al. A melanin-mediated cancer immunotherapy patch. Sci Immunol. 2017;2(17):eaan5692.
Ali AA, McCrudden CM, McCaffrey J, McBride JW, Cole G, Dunne NJ, et al. DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles. Nanomed Nanotechnol Biol Med. 2017;13(3):921–32.
Cole G, Ali AA, McErlean E, Mulholland EJ, Short A, McCrudden CM, et al. DNA vaccination via RALA nanoparticles in a microneedle delivery system induces a potent immune response against the endogenous prostate cancer stem cell antigen. Acta Biomater. 2019;96:480–90. https://doi.org/10.1016/j.actbio.2019.07.003.
Lee K, Kim JD, Lee CY, Her S, Jung H. A high-capacity, hybrid electro-microneedle for in-situ cutaneous gene transfer. Biomaterials. 2011;32(30):7705–10.
Pan J, Ruan W, Qin M, Long Y, Wan T, Yu K, et al. Intradermal delivery of STAT3 siRNA to treat melanoma via dissolving microneedles. Sci Rep. 2018;8(1):1–11.
Xu Q, Li X, Zhang P, Wang Y. Rapidly dissolving microneedle patch for synergistic gene and photothermal therapy of subcutaneous tumor. J Mater Chem B. 2020;8:4331–4339.
Bystrova S, Luttge R. Micromolding for ceramic microneedle arrays. MiEng. 2011;88(8):1681–4.
Gupta J, Gill HS, Andrews SN, Prausnitz MR. Kinetics of skin resealing after insertion of microneedles in human subjects. J Control Release. 2011;154(2):148–55.
Parker E, Rao M, Turner K, Meinhart C, MacDonald N. Bulk micromachined titanium microneedles. JMemS. 2007;16(2):289–95.
Jung PG, Lee TW, Oh DJ, Hwang SJ, Jung ID, Lee SM, et al. Nickel microneedles fabricated by sequential copper and nickel electroless plating and copper chemical wet etching. Sens Mater. 2008;20(1):45–53.
Park J-H, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104(1):51–66.
Han M, Hyun D-H, Park H-H, Lee SS, Kim C-H, Kim C. A novel fabrication process for out-of-plane microneedle sheets of biocompatible polymer. JMiMi. 2007;17(6):1184.
Wilke N, Mulcahy A, Ye S-R, Morrissey A. Process optimization and characterization of silicon microneedles fabricated by wet etch technology. Microelectron J. 2005;36(7):650–6.
Chabri F, Bouris K, Jones T, Barrow D, Hann A, Allender C, et al. Microfabricated silicon microneedles for nonviral cutaneous gene delivery. Br J Dermatol. 2004;150(5):869–77.
Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004;21(6):947–52.
Zhang Y, Brown K, Siebenaler K, Determan A, Dohmeier D, Hansen K. Development of lidocaine-coated microneedle product for rapid, safe, and prolonged local analgesic action. Pharm Res. 2012;29(1):170–7.
Park J-H, Choi S-O, Seo S, Choy YB, Prausnitz MR. A microneedle roller for transdermal drug delivery. Eur J Pharm Biopharm. 2010;76(2):282–9.
Chen Y, Chen BZ, Wang QL, Jin X, Guo XD. Fabrication of coated polymer microneedles for transdermal drug delivery. J Control Release. 2017;265:14–21.
Johnson AR, Caudill CL, Tumbleston JR, Bloomquist CJ, Moga KA, Ermoshkin A, et al. Single-step fabrication of computationally designed microneedles by continuous liquid interface production. PLoS One. 2016;11(9):e0162518.
Vecchione R, Coppola S, Esposito E, Casale C, Vespini V, Grilli S, et al. Electro-drawn drug-loaded biodegradable polymer microneedles as a viable route to hypodermic injection. Adv Funct Mater. 2014;24(23):3515–23.
Kirkby M, Hutton ARJ, Donnelly RF. Microneedle mediated transdermal delivery of protein, peptide and antibody based therapeutics: current status and future considerations. Pharm Res. 2020;37(6):117. https://doi.org/10.1007/s11095-020-02844-6.
O’Mahony C. Structural characterization and in-vivo reliability evaluation of silicon microneedles. BioMi. 2014;16(3):333–43.
Donnelly RF, Singh TRR, Tunney MM, Morrow DI, McCarron PA, O’Mahony C, et al. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm Res. 2009;26(11):2513–22.
Donnelly RF, Singh TRR, Alkilani AZ, McCrudden MT, O’Neill S, O’Mahony C, et al. Hydrogel-forming microneedle arrays exhibit antimicrobial properties: potential for enhanced patient safety. Int J Pharm. 2013;451(1–2):76–91.
Banks SL, Paudel KS, Brogden NK, Loftin CD, Stinchcomb AL. Diclofenac enables prolonged delivery of naltrexone through microneedle-treated skin. Pharm Res. 2011;28(5):1211–9.
Chen X, Fernando GJ, Crichton ML, Flaim C, Yukiko SR, Fairmaid EJ, et al. Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. J Control Release. 2011;152(3):349–55.
Gittard S, Narayan R, Jin C, Ovsianikov A, Chichkov B, Monteiro-Riviere N, et al. Pulsed laser deposition of antimicrobial silver coating on Ormocer® microneedles. Biofabrication. 2009;1(4):041001.
Gill HS, Prausnitz MR. Pocketed microneedles for drug delivery to the skin. JPCS. 2008;69(5–6):1537–41.
Ameri M, Fan SC, Maa Y-F. Parathyroid hormone PTH (1-34) formulation that enables uniform coating on a novel transdermal microprojection delivery system. Pharm Res. 2010;27(2):303–13.
Boehm RD, Miller PR, Daniels J, Stafslien S, Narayan RJ. Inkjet printing for pharmaceutical applications. Mater Today. 2014;17(5):247–52.
Lee HS, Ryu HR, Roh JY, Park J-H. Bleomycin-coated microneedles for treatment of warts. Pharm Res. 2017;34(1):101–12.
Boehm RD, Jaipan P, Skoog SA, Stafslien S, VanderWal L, Narayan RJ. Inkjet deposition of itraconazole onto poly (glycolic acid) microneedle arrays. Biointerphases. 2016;11(1):011008.
Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117(2):227–37.
McGrath MG, Vrdoljak A, O’Mahony C, Oliveira JC, Moore AC, Crean AM. Determination of parameters for successful spray coating of silicon microneedle arrays. Int J Pharm. 2011;415(1–2):140–9.
Chen X, Prow TW, Crichton ML, Jenkins DW, Roberts MS, Frazer IH, et al. Dry-coated microprojection array patches for targeted delivery of immunotherapeutics to the skin. J Control Release. 2009;139(3):212–20.
Boehm R, Miller P, Hayes S, Monteiro-Riviere N, Narayan R. Modification of microneedles using inkjet printing. AIP Adv. 2011;1(2):022139.
Invernale MA, Tang BC, York RL, Le L, Hou DY, Anderson DG. Microneedle electrodes toward an amperometric glucose-sensing smart patch. Adv Healthc Mater. 2014;3(3):338–42.
Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Del Rev. 2004;56(5):581–7.
Ronnander P, Simon L, Spilgies H, Koch A. Modelling the in-vitro dissolution and release of sumatriptan succinate from polyvinylpyrrolidone-based microneedles. Eur J Pharm Sci. 2018;125:54–63.
Chen M-C, Ling M-H, Kusuma SJ. Poly-γ-glutamic acid microneedles with a supporting structure design as a potential tool for transdermal delivery of insulin. Acta Biomater. 2015;24:106–16.
Hao Y, Chen Y, Lei M, Zhang T, Cao Y, Peng J, et al. Near-infrared responsive PEGylated gold nanorod and doxorubicin loaded dissolvable hyaluronic acid microneedles for human epidermoid cancer therapy. Adv Therapeutics. 2018;1(2):1800008.
Chen M-C, Lai K-Y, Ling M-H, Lin C-W. Enhancing immunogenicity of antigens through sustained intradermal delivery using chitosan microneedles with a patch-dissolvable design. Acta Biomater. 2018;65:66–75.
Migalska K, Morrow DI, Garland MJ, Thakur R, Woolfson AD, Donnelly RF. Laser-engineered dissolving microneedle arrays for transdermal macromolecular drug delivery. Pharm Res. 2011;28(8):1919–30.
Chen BZ, Ashfaq M, Zhu DD, Zhang XP, Guo XD. Controlled delivery of insulin using rapidly separating microneedles fabricated from Genipin-crosslinked gelatin. Macromol Rapid Commun. 2018;39(20):1800075.
Ito Y, Murakami A, Maeda T, Sugioka N, Takada K. Evaluation of self-dissolving needles containing low molecular weight heparin (LMWH) in rats. Int J Pharm. 2008;349(1–2):124–9.
Ito Y, Yoshimitsu J-I, Shiroyama K, Sugioka N, Takada K. Self-dissolving microneedles for the percutaneous absorption of EPO in mice. J Drug Target. 2006;14(5):255–61. https://doi.org/10.1080/10611860600785080.
Loizidou EZ, Williams NA, Barrow DA, Eaton MJ, McCrory J, Evans SL, et al. Structural characterisation and transdermal delivery studies on sugar microneedles: experimental and finite element modelling analyses. Eur J Pharm Biopharm. 2015;89:224–31.
Martin C, Allender CJ, Brain KR, Morrissey A, Birchall JC. Low temperature fabrication of biodegradable sugar glass microneedles for transdermal drug delivery applications. J Control Release. 2012;158(1):93–101.
Asmawi AA, Salim N, Ngan CL, Ahmad H, Abdulmalek E, Masarudin MJ, et al. Excipient selection and aerodynamic characterization of nebulized lipid-based nanoemulsion loaded with docetaxel for lung cancer treatment. Drug Deliv Transl Res. 2019;9(2):543–554.
Jin X, Zhu DD, Chen BZ, Ashfaq M, Guo XD. Insulin delivery systems combined with microneedle technology. Adv Drug Del Rev. 2018.
Larraneta E, Lutton RE, Woolfson AD, Donnelly RF. Microneedle arrays as transdermal and intradermal drug delivery systems: materials science, manufacture and commercial development. Mater Sci Eng R Rep. 2016;104:1–32.
Sabri AH, Ogilvie J, Abdulhamid K, Shpadaruk V, McKenna J, Segal J, et al. Expanding the applications of microneedles in dermatology. Eur J Pharm Biopharm. 2019;140:121–140.
Ita K. Dissolving microneedles for transdermal drug delivery: advances and challenges. Biomed Pharmacother. 2017;93:1116–27.
Liu S, M-n J, Y-s Q, Kamiyama F, Katsumi H, Sakane T, et al. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J Control Release. 2012;161(3):933–41.
Donnelly RF, Morrow DI, Singh TR, Migalska K, McCarron PA, O'Mahony C, et al. Processing difficulties and instability of carbohydrate microneedle arrays. Drug Dev Ind Pharm. 2009;35(10):1242–54.
Xie S, Li Z, Yu Z. Microneedles for transdermal delivery of insulin. J Drug Deliv Sci Technol. 2015;28:11–7.
Uddin MJ, Scoutaris N, Economidou SN, Giraud C, Chowdhry BZ, Donnelly RF, et al. 3D printed microneedles for anticancer therapy of skin tumours. Mater Sci Eng C. 2020;107:110248.
van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans) dermal drug and vaccine delivery. J Control Release. 2012;161(2):645–55.
Miller PR, Narayan RJ, Polsky R. Microneedle-based sensors for medical diagnosis. J Mater Chem B. 2016;4(8):1379–83.
Stoeber B, Liepmann D. Arrays of hollow out-of-plane microneedles for drug delivery. JMemS. 2005;14(3):472–9.
Ovsianikov A, Chichkov B, Mente P, Monteiro-Riviere N, Doraiswamy A, Narayan R. Two photon polymerization of polymer–ceramic hybrid materials for transdermal drug delivery. Int J Appl Ceram Technol. 2007;4(1):22–9.
McAllister DV, Wang PM, Davis SP, Park J-H, Canatella PJ, Allen MG, et al. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci. 2003;100(24):13755–60.
Laurent PE, Bourhy H, Fantino M, Alchas P, Mikszta JA. Safety and efficacy of novel dermal and epidermal microneedle delivery systems for rabies vaccination in healthy adults. Vaccine. 2010;28(36):5850–6.
Davis SP, Martanto W, Allen MG, Prausnitz MR. Hollow metal microneedles for insulin delivery to diabetic rats. ITBE. 2005;52(5):909–15.
Yung K, Xu Y, Kang C, Liu H, Tam K, Ko S, et al. Sharp tipped plastic hollow microneedle array by microinjection moulding. JMiMi. 2011;22(1):015016.
Moon SJ, Lee SS, Lee H, Kwon T. Fabrication of microneedle array using LIGA and hot embossing process. Microsyst Technol. 2005;11(4–5):311–8.
Lyon BJ, Aria AI, Gharib M. Fabrication of carbon nanotube—polyimide composite hollow microneedles for transdermal drug delivery. BioMi. 2014;16(6):879–86.
Gardeniers HJ, Luttge R, Berenschot EJ, De Boer MJ, Yeshurun SY, Hefetz M, et al. Silicon micromachined hollow microneedles for transdermal liquid transport. JMemS. 2003;12(6):855–62.
Khanna P, Luongo K, Strom JA, Bhansali S. Sharpening of hollow silicon microneedles to reduce skin penetration force. JMiMi. 2010;20(4):045011.
Perennes F, Marmiroli B, Matteucci M, Tormen M, Vaccari L, Di Fabrizio E. Sharp beveled tip hollow microneedle arrays fabricated by LIGA and 3D soft lithography with polyvinyl alcohol. JMiMi. 2006;16(3):473.
Lee K, Lee HC, Lee DS, Jung H. Drawing lithography: three-dimensional fabrication of an ultrahigh-aspect-ratio microneedle. Adv Mater. 2010;22(4):483–6.
Wang P-C, Wester BA, Rajaraman S, Paik S-J, Kim S-H, Allen MG eds. Hollow polymer microneedle array fabricated by photolithography process combined with micromolding technique. Engineering in medicine and biology society, 2009. EMBC 2009. Annual International Conference of the IEEE; 2009: IEEE.
Lhernould MS, Deleers M, Delchambre A. Hollow polymer microneedles array resistance and insertion tests. Int J Pharm. 2015;480(1–2):152–7.
Gittard SD, Miller PR, Boehm RD, Ovsianikov A, Chichkov BN, Heiser J, et al. Multiphoton microscopy of transdermal quantum dot delivery using two photon polymerization-fabricated polymer microneedles. Faraday Discuss. 2011;149:171–85.
Trautmann A, Roth G-L, Nujiqi B, Walther T, Hellmann R. Towards a versatile point-of-care system combining femtosecond laser generated microfluidic channels and direct laser written microneedle arrays. Microsyst Nanoeng. 2019;5(1):6.
Norman JJ, Brown MR, Raviele NA, Prausnitz MR, Felner EI. Faster pharmacokinetics and increased patient acceptance of intradermal insulin delivery using a single hollow microneedle in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2013;14(6):459–65.
Rapid pharmacokinetics of intradermal insulin administered using microneedles in type 1 diabetes subjects. Diabetes Technol Ther. 2011;13(4):451–6. https://doi.org/10.1089/dia.2010.0204.
Pettis RJ, Ginsberg B, Hirsch L, Sutter D, Keith S, McVey E, et al. Intradermal microneedle delivery of insulin lispro achieves faster insulin absorption and insulin action than subcutaneous injection. Diabetes Technol Ther. 2011;13(4):435–42. https://doi.org/10.1089/dia.2010.0184.
Waghule T, Singhvi G, Dubey SK, Pandey MM, Gupta G, Singh M, et al. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–58.
Baron N, Passave J, Guichardaz B, Cabodevila G. Investigations of development process of high hollow beveled microneedles using a combination of ICP RIE and dicing saw. Microsyst Technol. 2008;14(9):1475–80. https://doi.org/10.1007/s00542-008-0596-1.
Lee D-S, Li CG, Ihm C, Jung H. A three-dimensional and bevel-angled ultrahigh aspect ratio microneedle for minimally invasive and painless blood sampling. Sensors Actuators B Chem. 2018;255:384–90.
Martanto W, Choi Y, Joung Y-H, Allen MG, Prausnitz MR. Side-opening hollow microneedles for transdermal drug delivery. Atlanta: School of Chemical and Biomolecular Engineering, School of Electrical and Computer Engineering, Georgia Institute of Technology; 2005.
Donnelly RF, Morrow DI, McCrudden MT, Alkilani AZ, Vicente-Pérez EM, O'Mahony C, et al. Hydrogel-forming and dissolving microneedles for enhanced delivery of photosensitizers and precursors. PcPb. 2014;90(3):641–7.
Hardy JG, Larrañeta E, Donnelly RF, McGoldrick N, Migalska K, McCrudden MT, et al. Hydrogel-forming microneedle arrays made from light-responsive materials for on-demand transdermal drug delivery. Mol Pharm. 2016;13(3):907–14.
Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, et al. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci. 2015;112(27):8260–5.
Yang S, Wu F, Liu J, Fan G, Welsh W, Zhu H, et al. Phase-transition microneedle patches for efficient and accurate transdermal delivery of insulin. Adv Funct Mater. 2015;25(29):4633–41.
Donnelly RF, Singh TRR, Garland MJ, Migalska K, Majithiya R, McCrudden CM, et al. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv Funct Mater. 2012;22(23):4879–90.
Demir YK, Metin AÜ, Şatıroğlu B, Solmaz ME, Kayser V, Mäder K. Poly (methyl vinyl ether-co-maleic acid) – pectin based hydrogel-forming systems: gel, film, and microneedles. Eur J Pharm Biopharm. 2017;117:182–94. https://doi.org/10.1016/j.ejpb.2017.04.018.
Dardano P, Caliò A, Di Palma V, Bevilacqua MF, Di Matteo A, De Stefano L. A photolithographic approach to polymeric microneedles array fabrication. Materials. 2015;8(12):8661–73.
Yang SY, O'Cearbhaill ED, Sisk GC, Park KM, Cho WK, Villiger M, et al. A bio-inspired swellable microneedle adhesive for mechanical interlocking with tissue. Nat Commun. 2013;4:1702.
Larraneta E, Lutton RE, Brady AJ, Vicente-Pérez EM, Woolfson AD, Thakur RRS, et al. Microwave-assisted preparation of hydrogel-forming microneedle arrays for transdermal drug delivery applications. Macromol Mater Eng. 2015;300(6):586–95.
McCrudden MT, Alkilani AZ, Courtenay AJ, McCrudden CM, McCloskey B, Walker C, et al. Considerations in the sterile manufacture of polymeric microneedle arrays. Drug Deliv Transl Res. 2015;5(1):3–14.
Dorrani M, Garbuzenko OB, Minko T, Michniak-Kohn B. Development of edge-activated liposomes for siRNA delivery to human basal epidermis for melanoma therapy. J Control Release. 2016;228:150–8.
Alimardani V, Abolmaali SS, Borandeh S. Antifungal and antibacterial properties of graphene-based nanomaterials: a mini-review. J Nanostruct. 2019;9(3):402–13.
Farahavar G, Abolmaali SS, Gholijani N, Nejatollahi F. Antibody-guided nanomedicines as novel breakthrough therapeutic, diagnostic and theranostic tools. Biomater Sci. 2019;7(10):4000–16.
Larrañeta E, McCrudden MT, Courtenay AJ, Donnelly RF. Microneedles: a new frontier in nanomedicine delivery. Pharm Res. 2016;33(5):1055–73.
Farvadi F, Tamaddon A, Sobhani Z, Abolmaali SS. Polyionic complex of single-walled carbon nanotubes and PEG-grafted-hyperbranched polyethyleneimine (PEG-PEI-SWNT) for an improved doxorubicin loading and delivery: development and in vitro characterization. Artif Cells Nanomed Biotechnol. 2017;45(5):855–63. https://doi.org/10.1080/21691401.2016.1181642.
Abolmaali SS, Tamaddon A, Najafi H, Dinarvand R. Effect of l-histidine substitution on sol–gel of transition metal coordinated poly ethyleneimine: synthesis and biochemical characterization. J Inorg Organomet Polym Mater. 2014;24(6):977–87. https://doi.org/10.1007/s10904-014-0067-3.
Indermun S, Govender M, Kumar P, Choonara YE, Pillay V. Stimuli-responsive polymers as smart drug delivery systems: classifications based on carrier type and triggered-release mechanism. Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications, Volume 1. Elsevier; 2018. p. 43–58.
Lau KG, Hattori Y, Chopra S, O’Toole EA, Storey A, Nagai T, et al. Ultra-deformable liposomes containing bleomycin: in vitro stability and toxicity on human cutaneous keratinocyte cell lines. Int J Pharm. 2005;300(1–2):4–12.
Ugurel M, Schadendorf D, Fink W, Zimpfer-Rechner C, Thoelke A, Figl R, et al. Clinical phase II study of pegylated liposomal doxorubicin as second-line treatment in disseminated melanoma. Oncol Res Treat. 2004;27(6):540–4.
Leonard R, Williams S, Tulpule A, Levine A, Oliveros S. Improving the therapeutic index of anthracycline chemotherapy: focus on liposomal doxorubicin (Myocet™). Breast. 2009;18(4):218–24.
Frampton JE. Mifamurtide. Pediatr Drugs. 2010;12(3):141–53.
Barenholz YC. Doxil®—the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–34.
Choi YH, Han H-K. Nanomedicines: current status and future perspectives in aspect of drug delivery and pharmacokinetics. J Pharm Investig. 2018;48(1):43–60. https://doi.org/10.1007/s40005-017-0370-4.
Sahu S, Saraf S, Kaur CD, Saraf S. Biocompatible nanoparticles for sustained topical delivery of anticancer phytoconstituent quercetin. Pak J Biol Sci. 2013;16(13):601–9 Shri Rawatpura Sarkar Institute of Pharmacy, Kumhari, Durg, CG, India University Institute of Pharmacy, Pt. Ravishankar Shukla UniversityRaipur, 492010, CG, India.
Mostoufi H, Yousefi G, Tamaddon A-m, Firouzi O. Reversing multi-drug tumor resistance to paclitaxel by well-defined pH-sensitive amphiphilic polypeptide block copolymers via induction of lysosomal membrane permeabilization. Colloids Surf B Biointerfaces. 2019;174:17–27.
Sartor O. Eligard: leuprolide acetate in a novel sustained-release delivery system. Urology. 2003;61(2):25–31.
Weissig V, Pettinger TK, Murdock N. Nanopharmaceuticals (part 1): products on the market. Int J Nanomedicine. 2014;9:4357.
Revia RA, Zhang M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances. Mater Today. 2016;19(3):157–68. https://doi.org/10.1016/j.mattod.2015.08.022.
Moreira AF, Rodrigues CF, Jacinto TA, Miguel SP, Costa EC, Correia IJ. Microneedle-based delivery devices for cancer therapy: a review. Pharmacol Res. 2019;148:104438.
Rejhová A, Opattová A, Čumová A, Slíva D, Vodička P. Natural compounds and combination therapy in colorectal cancer treatment. Eur J Med Chem. 2018;144:582–94. https://doi.org/10.1016/j.ejmech.2017.12.039.
Yu W-D, Sun G, Li J, Xu J, Wang X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 2019;452:66–70. https://doi.org/10.1016/j.canlet.2019.02.048.
Eldar-Boock A, Polyak D, Scomparin A, Satchi-Fainaro R. Nano-sized polymers and liposomes designed to deliver combination therapy for cancer. Curr Opin Biotechnol. 2013;24(4):682–9.
Liao J, Li W, Peng J, Yang Q, Li H, Wei Y, et al. Combined cancer photothermal-chemotherapy based on doxorubicin/gold nanorod-loaded polymersomes. Theranostics. 2015;5(4):345.
Chen M-C, Lin Z-W, Ling M-H. Near-infrared light-activatable microneedle system for treating superficial tumors by combination of chemotherapy and photothermal therapy. ACS Nano. 2015;10(1):93–101.
Dong L, Li Y, Li Z, Xu N, Liu P, Du H, et al. Au nanocage-strengthened dissolving microneedles for chemo-photothermal combined therapy of superficial skin tumors. ACS Appl Mater Interfaces. 2018;10(11):9247–56. https://doi.org/10.1021/acsami.7b18293.
Chen M, Quan G, Wen T, Yang P, Qin W, Mai H, et al. Cold to hot: binary cooperative microneedle array amplified photo-immunotherapy for eliciting antitumor immunity and Abscopal effect. ACS Appl Mater Interfaces. 2020;12(29):32259–32269.
Hally C, Rodríguez-Amigo B, Bresolí-Obach R, Planas O, Nos J, Boix-Garriga E et al. Photodynamic therapy. Theranostics and Image Guided Drug Delivery 2018. p. 86–122.
Mahmoudi K, Garvey K, Bouras A, Cramer G, Stepp H, Raj JJ, et al. 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. JNO. 2019;141(3):595–607.
Mahmoudi K, Garvey KL, Bouras A, Cramer G, Stepp H, Jesu Raj JG, et al. 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. J Neuro-Oncol. 2019;141(3):595–607. https://doi.org/10.1007/s11060-019-03103-4.
Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18(3):175–96.
Farhood B, Najafi M, Mortezaee K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. 2019;234(6):8509–21.
Milling L, Zhang Y, Irvine DJ. Delivering safer immunotherapies for cancer. Adv Drug Del Rev. 2017;114:79–101.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. https://doi.org/10.1038/nrc3239.
Feng M, Xiong G, Cao Z, Yang G, Zheng S, Song X, et al. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017;407:57–65. https://doi.org/10.1016/j.canlet.2017.08.006.
Wolchok JD, Hodi FS, Weber JS, Allison JP, Urba WJ, Robert C, et al. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. ANYAS. 2013;1291(1):1–13.
Palucka K, Ueno H, Fay J, Banchereau J. Dendritic cells and immunity against cancer. J Intern Med. 2011;269(1):64–73.
Elamanchili P, Diwan M, Cao M, Samuel J. Characterization of poly (D, L-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine. 2004;22(19):2406–12.
Zaric M, Lyubomska O, Touzelet O, Poux C, Al-Zahrani S, Fay F, et al. Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly-D, L-lactide-co-glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS Nano. 2013;7(3):2042–55.
Liu M. DNA vaccines: a review. J Intern Med. 2003;253(4):402–10.
Lopes A, Vandermeulen G, Préat V. Cancer DNA vaccines: current preclinical and clinical developments and future perspectives. J Exp Clin Cancer Res. 2019;38(1):146.
Arya J, Prausnitz MR. Microneedle patches for vaccination in developing countries. J Control Release. 2016;240:135–41.
Kim Y-C, Quan F-S, Yoo D-G, Compans RW, Kang S-M, Prausnitz MR. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J Infect Dis. 2010;201(2):190–8.
Raphael AP, Crichton ML, Falconer RJ, Meliga S, Chen X, Fernando GJ, et al. Formulations for microprojection/microneedle vaccine delivery: structure, strength and release profiles. J Control Release. 2016;225:40–52.
Bachy V, Hervouet C, Becker PD, Chorro L, Carlin LM, Herath S, et al. Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proc Natl Acad Sci. 2013;110(8):3041–6.
Edens C, Collins ML, Goodson JL, Rota PA, Prausnitz MR. A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine. 2015;33(37):4712–8.
Liao J-F, Lee J-C, Lin C-K, Wei K-C, Chen P-Y, Yang H-W. Self-assembly DNA polyplex vaccine inside dissolving microneedles for high-potency intradermal vaccination. Theranostics. 2017;7(10):2593.
Münger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78(21):11451–60.
Schiller JT, Müller M. Next generation prophylactic human papillomavirus vaccines. The Lancet Oncology. 2015;16(5):e217–e25.
Yang B, Yang A, Peng S, Pang X, Roden RB, Wu T-C, et al. Co-administration with DNA encoding papillomavirus capsid proteins enhances the antitumor effects generated by therapeutic HPV DNA vaccination. Cell Biosci. 2015;5(1):35.
Bloy N, Buqué A, Aranda F, Castoldi F, Eggermont A, Cremer I, et al. Trial watch: naked and vectored DNA-based anticancer vaccines. Oncoimmunology. 2015;4(5):e1026531.
Huang D, Zhao D, Huang Y, Liang Z, Li Z eds. Microneedle roller electrode array (M-REA): a new tool for in vivo low-voltage electric gene delivery. Micro electro mechanical systems (MEMS), 2018 IEEE; 2018: IEEE.
Pan J, Ruan W, Qin M, Long Y, Wan T, Yu K, et al. Intradermal delivery of STAT3 siRNA to treat melanoma via dissolving microneedles. Sci Rep. 2018;8(1):1117.
Choi S-O, Kim YC, Park J-H, Hutcheson J, Gill HS, Yoon Y-K, et al. An electrically active microneedle array for electroporation. BioMi. 2010;12(2):263–73. https://doi.org/10.1007/s10544-009-9381-x.
Prausnitz MR. The effects of electric current applied to skin: a review for transdermal drug delivery. Adv Drug Del Rev. 1996;18(3):395–425.
Miller PR, Xiao X, Brener I, Burckel DB, Narayan R, Polsky R. Microneedle-based transdermal sensor for on-chip potentiometric determination of K+. Adv Healthc Mater. 2014;3(6):876–81.
Meric-Bernstam F, Mills GB. Overcoming implementation challenges of personalized cancer therapy. Nat Rev Clin Oncol. 2012;9(9):542.
Tran BQ, Miller PR, Taylor RM, Boyd G, Mach PM, Rosenzweig CN, et al. Proteomic characterization of dermal interstitial fluid extracted using a novel microneedle-assisted technique. J Proteome Res. 2018;17(1):479–85.
Mohan AMV, Windmiller JR, Mishra RK, Wang J. Continuous minimally-invasive alcohol monitoring using microneedle sensor arrays. Biosens Bioelectron. 2017;91:574–9. https://doi.org/10.1016/j.bios.2017.01.016.
Lee SJ, Yoon HS, Xuan X, Park JY, Paik S-J, Allen MG. A patch type non-enzymatic biosensor based on 3D SUS micro-needle electrode array for minimally invasive continuous glucose monitoring. Sensors Actuators B Chem. 2016;222:1144–51. https://doi.org/10.1016/j.snb.2015.08.013.
Zahn JD, deshmukh A, Pisano AP, Liepmann D. Continuous on-chip micropumping for microneedle enhanced drug delivery. BioMi. 2004;6(3):183–90. https://doi.org/10.1023/B:BMMD.0000042047.83433.96.
Yan X, Li H, Zheng W, Su X. Visual and fluorescent detection of tyrosinase activity by using a dual-emission ratiometric fluorescence probe. AnaCh. 2015;87(17):8904–9. https://doi.org/10.1021/acs.analchem.5b02037.
Rao AR, Sindhuja HN, Dharmesh SM, Sankar KU, Sarada R, Ravishankar GA. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J Agric Food Chem. 2013;61(16):3842–51. https://doi.org/10.1021/jf304609j.
Ciui B, Martin A, Mishra RK, Brunetti B, Nakagawa T, Dawkins TJ, et al. Wearable wireless tyrosinase bandage and microneedle sensors: toward melanoma screening. Adv Healthc Mater. 2018;7(7):1701264.
Rubin EH, Gilliland DG. Drug development and clinical trials—the path to an approved cancer drug. Nat Rev Clin Oncol. 2012;9(4):215.
Jonas O, Landry HM, Fuller JE, Santini JT, Baselga J, Tepper RI, et al. An implantable microdevice to perform high-throughput in vivo drug sensitivity testing in tumors. Sci Transl Med. 2015;7(284):284ra57.
Haq M, Smith E, John DN, Kalavala M, Edwards C, Anstey A, et al. Clinical administration of microneedles: skin puncture, pain and sensation. BioMi. 2009;11(1):35–47.
Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human subjects. Clin J Pain. 2008;24(7):585.
Noh Y-W, Kim T-H, Baek J-S, Park H-H, Lee SS, Han M, et al. In vitro characterization of the invasiveness of polymer microneedle against skin. Int J Pharm. 2010;397(1–2):201–5.
Al-Kasasbeh R, Brady AJ, Courtenay AJ, Larrañeta E, McCrudden MT, O’Kane D, et al. Evaluation of the clinical impact of repeat application of hydrogel-forming microneedle array patches. Drug Deliv Transl Res. 2020;10:690–705.
Zahn JD, Pisano AP, Liepmann D. Continuous on-chip micropumping for microneedle enhanced drug delivery. BioMi. 2004;6(3):183–90.
Acknowledgments
This work supported by grant from Shiraz University of Medical Sciences (SUMS), Shiraz, Iran. The authors wish to thank Mr. H. Argasi at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for his invaluable assistance in editing this manuscript.
Funding
This article is a part of Mr. Vahid Alimardani thesis funded by Shiraz University of Medical Sciences under supervision of Dr. Samirasadat Abolmaali.
Author information
Authors and Affiliations
Contributions
All the authors have significantly contributed to the concept and writing of manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alimardani, V., Abolmaali, S.S., Tamaddon, A.M. et al. Recent advances on microneedle arrays-mediated technology in cancer diagnosis and therapy. Drug Deliv. and Transl. Res. 11, 788–816 (2021). https://doi.org/10.1007/s13346-020-00819-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00819-z