Abstract
Chemical injury by alkali burn is a major cause of corneal blindness in the clinical setting. Current management advocates multiple therapies aimed to prevent inflammation, initiate quick re-epithelialization, arrest the fibrosis, and avoid dry eye and pain by using bandage contact lenses. We hypothesized sustained delivery of the anti-inflammatory, antifibrotic drug pirfenidone through vitamin E-loaded contact lenses as a logical single approach to counter the pathology involved. Vitamin E particles were created in situ in commercial silicon hydrogel contact lenses by soaking the lenses in a vitamin E-ethanol solution. The vitamin E-laden lenses were then placed into pirfenidone-saline solution to load the drug into the lens. The contact lenses were evaluated by both in vitro and in vivo means. For in vitro, lenses were placed into 3 mL of saline solution. The concentration of pirfenidone released was measured by UV-vis spectrophotometry. The contact lenses were implanted in rabbit eyes following the alkali burn; the drug availability in the aqueous humor was evaluated by HPLC at various time points 10 min, 30 min, 2 h, and 3 h; and gene expression of inflammatory cytokines IL-1β, TNF-α, and TGF-β1 was evaluated in the cornea at the end of the study period. In another group of rabbits inflicted with alkali injury, the corneas were graded after 7 days of contact lens implantation with and without pirfenidone. A mathematical model was developed for delivery of the drug to the cornea and aqueous humor after a contact lens is inserted in the eye. The model was validated with experimental data and used to determine the bioavailability both for contact lenses and eye drops. In vitro release of unmodified commercial contact lenses saw a release time of approximately 20 min, with a partition coefficient of 2.68 ± 0.06. The release of pirfenidone from 20% vitamin E-loaded lenses saw a release time of approximately 80 min, with a partition coefficient of 4.20 ± 0.04. In vivo, the drug was available in the aqueous humor for up to 3 h. Gene expression of inflammatory cytokine IL-β1 and profibrotic growth factor TGF-β1 was significantly suppressed in corneas treated with pirfenidone contact lenses. A week after the alkali burn, the eyes with pirfenidone contact lenses showed significant improvement in corneal haze in comparison to the control eyes. About 50% of the drug loaded in the lens reached the aqueous humor compared to 1.3% with eye drops. Vitamin E-loaded contact lenses serve as a suitable platform for delivery of pirfenidone following alkali burn in rabbit eyes; positive pre-clinical outcome identifies it as promising therapy for addressing corneal inflammation and fibrosis. The bioavailability is about 40-fold higher for contact lenses compared to that for eye drops.









Similar content being viewed by others
Change history
22 September 2018
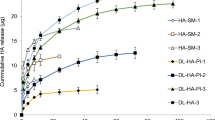
In the original article the typesetter made several errors. Figures 7 and 9 are incorrect. Following are the correct figures:
References
Clare G, Suleman H, Bunce C, Dua H. Amniotic membrane transplantation for acute ocular burns. Cochrane Database Syst Rev. 2012;9:CD009379. https://doi.org/10.1002/14651858.CD009379.
Wagoner MD. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol. 1997;41:275–313.
Maher TM. Pirfenidone in idiopathic pulmonary fibrosis. Drugs Today (Barc). 2010;46:473–82. https://doi.org/10.1358/dot.2010.46.7.1488336.
Khanum BNMK, Guha R, Sur VP, Nandi S, Basak SK, Konar A, et al. Pirfenidone inhibits post-traumatic proliferative vitreoretinopathy. Eye. 2017;31:1317–28. https://doi.org/10.1038/eye2017.21.
Zhong H, Sun G, Lin X, Wu K, Yu M. Evaluation of pirfenidone as a new postoperative antiscarring agent in experimental glaucoma surgery. Invest Ophthalmol Vis Sci. 2011;16:3136–42. https://doi.org/10.1167/iovs.10-6240.
Sun G, Lin X, Zhong H, Yang Y, Qiu X, Ye C, et al. Pharmacokinetics of pirfenidone after topical administration in rabbit eye. Mol Vis. 2011;17:2191–6.
Bourlais CL, Acar L, Zia H, Sado PA, Needham T, Leverge R. Ophthalmic drug delivery systems. Prog Retin Eye Res. 1998;17:33–58.
Mitra AK. Ophthalmic drug delivery systems. New York: Marcel Dekker Inc; 1993. p. 60.
McNamara NA, Polse KA, Brand RJ, Graham AD, Chan JS, McKenney CD. Tear mixing under a soft contact lens: effects of lens diameter. Am J Ophthalmol. 1999;127:659–65.
Gause S, Hsu KH, Shafor C, Dixon P, Powell KC, Chauhan A. Mechanistic modeling of ophthalmic drug delivery to the anterior chamber by eye drops and contact lenses. Adv Colloid Interf Sci. 2016;233:139–54. https://doi.org/10.1016/j.cis.2015.08.002.
Li C-C, Chauhan A. Modeling ophthalmic drug delivery by soaked contact lenses. Ind Eng Chem Res. 2006;45:3718–34. https://doi.org/10.1021/ie0507934.
Peng C-C, Kim J, Chauhan A. Ivery of hydrophilic drugs from silicone-hydrogel contact lenses containing vitamin E diffusion barriers. Biomaterials. 2010;31:4032–47. https://doi.org/10.1016/j.biomaterials.2010.01.113.
Peng C-C, Chauhan A. Extended cyclosporine delivery by silicone-hydrogel contact lenses. J Control Release. 2011;154:267–74. https://doi.org/10.1016/j.jconrel.2011.06.028.
Peng C-C, Burke MT, Chauhan A. Transport of topical anesthetics in vitamin E loaded silicone hydrogel contact lenses. Langmuir. 2012;28:1478–87. https://doi.org/10.1021/la203606z.
Hsu K, Fentzke R, Chauhan A. Feasibility of corneal drug delivery of cysteamine using vitamin E modified silicone hydrogel contact lenses. Eur J Pharm Biopharm. 2013;85(3 PtA):531–40. https://doi.org/10.1016/j.ejpb.2013.04.017.
Paradiso P, Serro AP, Saramago B, Colaço R, Chauhan A. Controlled release of antibiotics from vitamin E-loaded silicone-hydrogel contact lenses. J Pharm Sci. 2016;105:1164–72. https://doi.org/10.1016/S0022-3549(15)00193-8.
Fantes EE, Hanna KD, Waring GO 3rd, Pouliquen Y, Thompson KP, et al. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990;108:665–75.
Chan T, Payor S, HoldenB A. Corneal thickness profiles in rabbits using an ultrasonic pachometer. Invest Ophthalmol Vis Sci. 1983;24(10):1408–10.
Zhang W, Prausnitz M, Edwards A. Model of transient drug diffusion across cornea. J Control Release. 2004;99(2):241–58. https://doi.org/10.1016/j.jconrel.2004.07.001. ISSN 0168–3659
Toris CB, Zhan G-L, McLaughlin MA. Effects of Brinzolamide on aqueous humor dynamics in monkeys and rabbits. J Ocul Pharmacol Ther 2004;19:397–404. doi: https://doi.org/10.1089/108076803322472962.
Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–21.
Chowdhury S, Guha R, Trivedi R, Kompella UB, Konar A, Hazra S. Pirfenidone nanoparticles improve corneal wound healing and prevent scarring following alkali burn. PLoS One. 2013;8:e70528. https://doi.org/10.1371/journal.pone.0070528.
Yang M, Yang Y, Lei M, Ye C, Zhao C, Xu J, et al. Experimental studies on soft contact lenses for controlled ocular delivery of pirfinedone: in vitro and in vivo. Drug Delivery. 2016;23:3538–43. https://doi.org/10.1080/10717544.2016.1204570.
Sotozono C, He MJ, Kita M, Imanshi J, Kinoshita S. Cytokine expression in alkali burned cornea. Curr Eye Res. 1997;16:670–6. https://doi.org/10.1076/ceyr.16.7.670.5057.
Cade F, Paschalis EI, Regatieri CV, Vavvas DG, Dana R, Dohlman CH. Alkali burn to the eye: protection using TNF-α inhibition. Cornea. 2014;33:382–9. https://doi.org/10.1097/ICO.0000000000000071.
Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91:326–35. https://doi.org/10.1016/j.exer.2010.06.021.
Macías-Barragán J, Sandoval-Rodríguez A, Navarro-Partida J, Armendáriz-Borunda J. The multifaceted role of pirfenidone and its novel targets. Fibrogenesis Tissue Repair. 2010;3:16. https://doi.org/10.1186/1755-1536-3-16.
Den S, Sotozono C, Kinoshita S, Ikeda T. Efficacy of early systemic betamethasone or cyclosporin A after corneal alkali injury via inflammatory cytokine reduction. Acta Ophthalmol Scand. 2004;82:195–9. https://doi.org/10.1046/j.1600-0420.2004.00229.x.
Creech JL, Chauhan A, Radke CJ. Dispersive mixing in the posterior tear film under a soft contact lens. Ind Eng Chem Res. 2001;40:3015–26. https://doi.org/10.1021/ie000596z.
Zhu H, Chauhan A. A mathematical model for tear drainage through the canaliculi. Curr Eye Res. 2005;30:621–30. https://doi.org/10.1080/02713680590968628.
Edwards A, Prausnitz M. Fiber matrix model of sclera and corneal stroma for drug delivery to the eye. AICHE J. 1998;4:214–25. https://doi.org/10.1002/aic.690440123.
Peng C-C, Chauhan A. Ion transport in silicone hydrogel contact lenses. J Membr Sci. 2012;399-400:95–105. https://doi.org/10.1016/j.memsci.2012.01.039.
Watsky M, Jablonski M, Edelhauser H. Comparison of conjunctival and corneal surface areas in rabbit and human. J Curr Eye Res. 1988;7:483–6. https://doi.org/10.3109/02713688809031801.
Prausnitz M, Edwards A. Predicted permeability of the cornea to topical drugs. J Pharm Res. 2001;18:1497–508.
Prausntiz M, Noonan J. Permeability of cornea, sclera, and conjuctiva: a literature analysis for drug delivery to the eye. J Pharm Sci. 1998;87:1479–88.
Acknowledgements
We acknowledge the fund received from the Department of Science and Technology, Govt of India; West Bengal University of Animal & Fishery Sciences; CSIR-IICB; and the Dept of Chemical Engineering, University of Florida, for providing the necessary infrastructure.
Nomenclature
α, aspect ratio of barrier; φ, vitamin E loading in lens; φ∗, absorbed vitamin E in lens; τ, time scales of pirfenidone release of vitamin E loaded; τ0, time scales of pirfenidone release of control lens; Aconjunctiva, area of conjunctiva; As, surface area of contact lens; Caq, concentration of pirfenidone in aqueous humor/cornea system; Cg, concentration of pirfenidone in contact lens; Cg, f, concentration of pirfenidone in contact lens after loading; Cg, i, concentration of pirfenidone in contact lens after loading; Cl, f concentration of pirfenidone in loading solution; C0, concentration at time zero for tear film after the elution of the eye drop; Cr, concentration of pirfenidone in release medium; Cr, f, final concentration of release medium; Ctear, concentration of pirfenidone in tear film; D, diffusion coefficient of gel; f, fraction of drug released from the lens that reaches the cornea; feye drops, bioavailability of eye drops; h, half thickness of contact lens; hc, thickness of cornea; hPOLTF, thickness of tear film; ja, flux of pirfenidone into cornea; jlens, flux of pirfenidone from lens; Kg, partition coefficient of pirfenidone in contact lens; Kconjunctiva, permeability of pirfenidone in conjunctiva; Kcornea, permeability of pirfenidone in cornea; Mo, total mass of pirfenidone in an eye drop; qaq, volumetric drainage rate from aqueous humor/cornea system; qtear, drainage rate of tear film; t, time; td, diffusion time of pirfenidone in contact lens; td, aq, diffusion time of pirfenidone in aqueous humor; td, tear, diffusion time of pirfenidone in tear film; tq, aq, time scale of drainage from aqueous humor; tq, tear, time scale of drainage from tear film; Vaq, volume of aqueous humor and cornea; Vg, volume of contact lens; Vr, volume of release medium; Vtear, volume of tear film; y, axis of thickness
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dixon, P., Ghosh, T., Mondal, K. et al. Controlled delivery of pirfenidone through vitamin E-loaded contact lens ameliorates corneal inflammation. Drug Deliv. and Transl. Res. 8, 1114–1126 (2018). https://doi.org/10.1007/s13346-018-0541-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-018-0541-5