Abstract
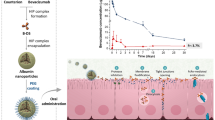
The present investigation deals with preparation and characterization of anti-migraine zolmitriptan (ZMT) nanostructured polymeric carriers for nose to brain drug targeting. The drug-loaded colloidal nanocarriers of ZMT were prepared by modified ionic gelation of cationic chitosan with anionic sodium tripolyphosphate and characterized for particle size, zeta potential, and entrapment efficiency. Further, in order to investigate nose to brain drug targeting, biodistribution, and brain kinetics studies were performed using 99mtechnetium radiolabeled nanocarriers (99mTc-ZMTNP) in Swiss albino mice. The results were compared with intranasal pure drug solution (99mTc-ZMT) and intravenous nanocarriers (99mTc-ZMTNP). A single photon emission computerized tomography (SPECT) radioimaging studies were also carried out to visualize and confirm brain uptake of nanocarriers. The optimized nanocarriers showed particle size of 161 nm, entrapment efficiency of 80.6%, and zeta potential of + 23.7 mV. The pharmacokinetic parameters, Cmax, and AUC0-∞ values for ZMT concentration in the brain expressed as percent radioactivity per gram of brain in intranasal and intravenous route of administration were calculated. The brain Cmax and AUC0-∞ values found in three groups, intranasal 99mTc-ZMTNP, intranasal 99mTc-ZMT, and intravenous 99mTc-ZMTNP were (0.427 and 1.889), (0.272 and 0.7157), and (0.204 and 0.9333), respectively. The higher Cmax values of intranasal 99mTc-ZMTNP suggests better brain uptake as compared to other routes of administration. The significant higher values of nose to brain targeting parameters namely, drug targeting index (5.57), drug targeting efficiency (557.08%), and nose to brain drug direct transport (82.05%) confirmed drug targeting to brain via nasal route. The coupled bimodal SPECT-CT scintigrams confirm the brain uptake of intranasal 99mTc-ZMTNP demonstrating major radioactivity accumulation in brain. This study conclusively demonstrated the greater uptake of ZMT-loaded nanocarriers by nose to brain drug targeting, which proves promising drug delivery system.




Similar content being viewed by others
References
Wang X, Chi N, Tang X. Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur J Pharm Biopharm. 2008;70(3):735–40. https://doi.org/10.1016/j.ejpb.2008.07.005.
Dhuria SV, Hanson LR, Frey WH. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654–73. https://doi.org/10.1002/jps.21924.
Bigal ME, Krymchantowski AV, Hargreaves R. The triptans. Expert Rev Neurother. 2009;9(5):649–59. https://doi.org/10.1586/ern.09.15.
Charlesworth B, Dowson AJ. Review of zolmitriptan and its clinical applications in migraine. Expert Opin Pharmacother. 2002;3(7):993–1005. https://doi.org/10.1517/14656566.3.7.993.
Uemura N, Onishi T, Mitaniyama A, Kaneko T, Ninomiya K, Nakamura K, et al. Bioequivalence and rapid absorption of zolmitriptan nasal spray compared with oral tablets in healthy Japanese subjects. Clin Drug Investig. 2005;25(3):199–208. https://doi.org/10.2165/00044011-200525030-00006.
Goadsby PJ, Yates R. Zolmitriptan intranasal: a review of the pharmacokinetics and clinical efficacy. Headache: J Head Face Pain. 2006;46(1):138–49. https://doi.org/10.1111/j.1526-4610.2006.00301.x.
Dixon R, Warrander A. The clinical pharmacokinetics of zolmitriptan. Cephalalgia. 1997;17(18):15–20. https://doi.org/10.1177/0333102497017S1803.
Rinaudo M. Chitin and chitosan: properties and applications. Prog Polym Sci. 2006;31(7):603–32. https://doi.org/10.1016/j.progpolymsci.2006.06.001.
Li J, Du Y, Yang J, Feng T, Li A, Chen P. Preparation and characterisation of low molecular weight chitosan and chito-oligomers by a commercial enzyme. Polym Degrad Stab. 2005;87(3):441–8. https://doi.org/10.1016/j.polymdegradstab.2004.09.008.
Illum L. Nasal drug delivery - possibilities, problems and solutions. J Cont Rel. 2003;87(1):187–98. https://doi.org/10.1016/S0168-3659(02)00363-2.
Chaudhari P. Preclinical animal model and non-invasive imaging in apoptosis. In: Proteases in apoptosis: pathways, protocols and translational advances: Springer International Publishing; 2015. p. 203–37.
Chapman SE, Diener JM, Sasser TA, Correcher C, González AJ, Van Avermaete T, et al. Dual tracer imaging of SPECT and PET probes in living mice using a sequential protocol. Am J Nucl Med Mol Imaging. 2012;2(4):405–14.
Koukaras EN, Papadimitriou SA, Bikiaris DN, Froudakis GE. Insight on the formation of chitosan nanoparticles through ionotropic gelation with tripolyphosphate. Mol Pharm. 2012;9(10):2856–62. https://doi.org/10.1021/mp300162j.
Mandlik SK, Ranpise NS. Implementation of experimental design methodology in preparation and characterization of zolmitriptan loaded chitosan nanoparticles. Int Cur Pharm J. 2017;6(3):16–22.
Patel S, Chavhan S, Soni H, Babbar AK, Mathur R, Mishra AK, et al. Brain targeting of risperidone-loaded solid lipid nanoparticles by intranasal route. J Drug Target. 2011;19(6):468–74. https://doi.org/10.3109/1061186X.2010.523787.
Saha GB. Methods of radiolabeling. Physics and radiobiology of nuclear medicine. New York, NY: Springer-Verlag. 1993:100–106.
Babbar AK, Singh AK, Goel HC, Chauhan UP, Sharma RK. Evaluation of 99m Tc-labeled photosan-3, a hematoporphyrin derivative, as a potential radiopharmaceutical for tumor scintigraphy. Nucl Med Biol. 2000;27(4):419–26. https://doi.org/10.1016/S0969-8051(00)00092-5.
Shadab MD, Kumar M, Baboota S, Sahni JK, Ali J. Preparation, characterization and evaluation of bromocriptine loaded chitosan nanoparticles for intranasal delivery. 2012;Sci Tech Adv Mater, 4(9):949–60. https://doi.org/10.1166/sam.2012.1380.
Kumar M, Misra A, Mishra AK, Mishra P, Pathak K. Mucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting. J Drug Target. 2008;16(10):806–14. https://doi.org/10.1080/10611860802476504.
Wang F, Jiang X, Lu W. Profiles of methotrexate in blood and CSF following intranasal and intravenous administration to rats. Int J Pharm. 2003;263(1):1–7. https://doi.org/10.1016/S0378-5173(03)00341-7.
Sherry Chow HH, Chen Z, Matsuura GT. Direct transport of cocaine from the nasal cavity to the brain following intranasal cocaine administration in rats. J Pharm Sci. 1999;88(8):754–8. https://doi.org/10.1021/js9900295.
Zhang Q, Jiang X, Jiang W, Lu W, Su L, Shi Z. Preparation of nimodipine-loaded microemulsion for intranasal delivery and evaluation on the targeting efficiency to the brain. Int J Pharm. 2004;275(1):85–96. https://doi.org/10.1016/j.ijpharm.2004.01.039.
Pathak P, Dhawan V, Magarkar A, Danne R, Govindarajan S, Ghosh S, et al. Design of cholesterol arabinogalactan anchored liposomes for asialoglycoprotein receptor mediated targeting to hepatocellular carcinoma: in silico modeling, in vitro and in vivo evaluation. Int J Pharm. 2016;509(1):149–58. https://doi.org/10.1016/j.ijpharm.2016.05.041.
Magota K, Kubo N, Kuge Y, Nishijima KI, Zhao S, Tamaki N. Performance characterization of the Inveon preclinical small-animal PET/SPECT/CT system for multimodality imaging. Eur J Nucl Med Mol Imaging. 2011;38(4):742–52. https://doi.org/10.1007/s00259-010-1683-y.
Thode K, Müller RH, Kresse M. Two time window and multiangle photon correlation spectroscopy size and zeta potential analysis- highly sensitive rapid assay for dispersion stability. J Pharm Sci. 2000;89(10):1317–24. https://doi.org/10.1002/1520-6017(200010)89:10<1317::AID-JPS9>3.0.CO;2-G.
Vila A, Sánchez A, Janes K, Behrens I, Kissel T, Jato JL, et al. Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice. Eur J Pharm Biopharm. 2004;57(1):123–31. https://doi.org/10.1016/j.ejpb.2003.09.006.
Gan Q, Wang T, Cochrane C, McCarron P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf B. 2005;44(2):65–73. https://doi.org/10.1016/j.colsurfb.2005.06.001.
Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60(4):110–1.
Banerjee T, Mitra S, Singh AK, Sharma RK, Maitra A. Preparation, characterization and biodistribution of ultrafine chitosan nanoparticles. Int J Pharm. 2002;243(1):93–105. https://doi.org/10.1016/S0378-5173(02)00267-3.
Charlton ST, Davis SS, Illum L. Evaluation of bioadhesive polymers as delivery systems for nose to brain delivery: in vitro characterisation studies. J Cont Rel. 2007;118(2):225–34. https://doi.org/10.1016/j.jconrel.2006.12.014.
Vllasaliu D, Exposito-Harris R, Heras A, Casettari L, Garnett M, Illum L, et al. Tight junction modulation by chitosan nanoparticles: comparison with chitosan solution. Int J Pharm. 2010;400(1):183–93. https://doi.org/10.1016/j.ijpharm.2010.08.020.
Pardeshi CV, Belgamwar VS. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood–brain barrier: an excellent platform for brain targeting. Expert Opin Drug Deliv. 2013;10(7):957–72. https://doi.org/10.1517/17425247.2013.790887.
Thorne RG, Frey WH. Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin Pharmacokinet. 2001;40(12):907–46. https://doi.org/10.2165/00003088-200140120-00003.
Mistry A, Stolnik S, Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm. 2009;379(1):146–57. https://doi.org/10.1016/j.ijpharm.2009.06.019.
Acknowledgements
The authors are grateful to Cipla Ltd., Mumbai, India, for providing a gift sample of ZMT. Authors are thankful to Savitribai Phule Pune University, India for providing financial support in the form of a research grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mandlik, S.K., Ranpise, N.S., Mohanty, B.S. et al. A coupled bimodal SPECT-CT imaging and brain kinetics studies of zolmitriptan-encapsulated nanostructured polymeric carriers. Drug Deliv. and Transl. Res. 8, 797–805 (2018). https://doi.org/10.1007/s13346-017-0474-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-017-0474-4