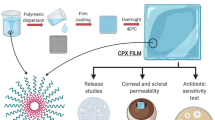
Abstract
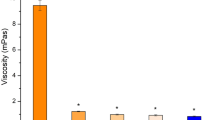
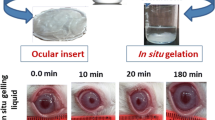
The objective of this research was to develop and evaluate an ocular insert for the controlled drug delivery of moxifloxacin which could perhaps be used in the treatment of corneal keratitis or even bacterial endophthalmitis. We have evaluated the ex vivo ocular diffusion of moxifloxacin through rabbit cornea, both fresh and preserved under different conditions. Histological studies were also carried out. Subsequently, drug matrix inserts were prepared using bioadhesive polymers. The inserts were evaluated for their physicochemical parameters. Ophthalmic ex vivo permeation of moxifloxacin was carried out with the most promising insert. The formulate insert was thin and provided higher ocular diffusion than commercial formulations. Ocular diffusion studies revealed significant differences between fresh and frozen corneas. Histological examinations also showed differences in the thickness of stroma between fresh and frozen corneas. The ophthalmic insert we have developed allows a larger quantity of moxifloxacin to permeate through the cornea than existing commercial formulations of the drug. Ocular delivery of moxifloxacin with this insert could be a new approach for the treatment of eye diseases.





Similar content being viewed by others
Abbreviations
- HPMC:
-
Hydroxypropyl methylcellulose 4500
- MOX:
-
Moxifloxacin
- PBS:
-
Phosphate buffered solution
- PEG:
-
Polyethylene glycol
- PVP-K30:
-
Polyvinylpyrrolidone K30
References
Souza JG, Dias K, Pereira TA, Bernardi DS, Lopez RFV. Topical delivery of ocular therapeutics: carrier systems and physical methods. J Pharm Pharmacol. 2014;66:507–30.
Vandervoort J, Ludwig A. Ocular drug delivery: nanomedicine applications. Nanomedicine. 2007;2:11–21.
Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12:348–60.
Mundada AS, Shrikhande BK. Design and evaluation of soluble ocular drug insert for controlled release of ciprofloxacin hydrochloride. Drug Dev Ind Pharm. 2006;32:443–8.
Mundada AS, Shrikhande BK. Formulation and evaluation of ciprofloxacin hydrochloride soluble ocular drug insert. Curr Eye Res. 2008;33:469–75.
Rathore K, Nema R. Review on ocular inserts. Int J PharmTech Res. 2009;1:164–9.
Rathore K, Nema R. An insight into ophthalmic drug delivery system. Int J Pharm Sci Drug Res. 2009;1:1–5.
Kampougeris G, Antoniadou A, Kavouklis E, Chryssouli Z, Giamarellou H. Penetration of moxifloxacin into the human aqueous humour after oral administration. Br J Ophthalmol. 2005;89:628–31.
Hariprasad SM, Shah GK, Mieler WF, Feiner L, Blinder KJ, Holekamp NM, et al. Vitreous and aqueous penetration of orally administered moxifloxacin in humans. Arch Ophthalmol. 2006;124:178.
Nam KY, Lee SJ, Kim JY. Systemic moxifloxacin in Streptococcus viridans endophthalmitis. Ocul Immunol Inflamm. 2017:1–7.
Yannuzzi NA, Si N, Relhan N, et al. Endophthalmitis after clear corneal cataract surgery: outcomes over two decades. Am J Ophthalmol. 2017;174:155–9.
Woodcock JM, Andrews JM, Boswell FJ, Brenwald NP, Wise R. In vitro activity of BAY 12-8039, a new fluoroquinolone. Antimicrob Agents Chemother. 1997;41:101–6.
Tyson SL, Bailey R, Roman JS, Zhan T, Hark LA, Haller JA. Clinical outcomes after injection of a compounded pharmaceutical for prophylaxis after cataract surgery. Curr Opin Ophthalmol. 2017;28:73–80.
Langlois M-H, Montagut M, Dubost J-P, Grellet J, Saux M-C. Protonation equilibrium and lipophilicity of moxifloxacin. J Pharm Biomed Anal. 2005;37:389–93.
Biedenbach DJ, Jones RN. The comparative antimicrobial activity of levofloxacin tested against 350 clinical isolates of streptococci. Diagn Microbiol Infect Dis. 1996;25:47–51.
Dalhoff A, Petersen U, Endermann R. In vitro activity of BAY 12-8039, a new 8-methoxyquinolone. Chemotherapy. 1996;42:410–25.
Davis R, Bryson HM. Levofloxacin. Drugs. 1994;47:677–700.
Pawar PK, Katara R, Majumdar DK. Design and evaluation of moxifloxacin hydrochloride ocular inserts. Acta Pharma. 2012;62:93–104.
Kalevar V. Donor corneae for preservation. A modified dissection technique. Br J Ophthalmol. 1968;52:695.
Majumdar S, Hingorani T, Srirangam R. Evaluation of active and passive transport processes in corneas extracted from preserved rabbit eyes. J Pharm Sci. 2010;99:1921–30.
Fu RC-C, Lidgate DM. In vitro rabbit corneal permeability study of ketorolac, tromethamine, a non-steroidal anti-inflammatory agent. Drug Dev Ind Pharm. 1986;12:2403–30.
Majumdar S, Hingorani T, Srirangam R, Gadepalli RS, Rimoldi JM, Repka MA. Transcorneal permeation of L- and D-aspartate ester prodrugs of acyclovir: delineation of passive diffusion versus transporter involvement. Pharm Res. 2009;26:1261–9.
Ahuja M, Dhake AS, Majumdar DK. Effect of formulation factors on in-vitro permeation of diclofenac from experimental and marketed aqueous eye drops through excised goat cornea. Yakugaku Zasshi. 2006;126:1369–75.
Pawar PK, Majumdar DK. Effect of formulation factors on in vitro permeation of moxifloxacin from aqueous drops through excised goat, sheep, and buffalo corneas. AAPS PharmSciTech. 2006;7:E1–6.
Srinivas N, Narasu L, Shankar BP, Mullangi R. Development and validation of a HPLC method for simultaneous quantitation of gatifloxacin, sparfloxacin and moxifloxacin using levofloxacin as internal standard in human plasma: application to a clinical pharmacokinetic study. Biomed Chromatogr. 2008;22:1288–95.
Rodríguez IC, Cerezo A, Salem II. Sistemas de liberación bioadhesivos. ARs Pharm. 2000;1:115–28.
Ramkanth S, Chetty C. Design and evaluation of diclofenac sodium ocusert. PharmTech Res. 2009;1:1219–23.
Balaguer-Fernández C, Padula C, Femenía-Font A, Merino V, Santi P, López-Castellano A. Development and evaluation of occlusive systems employing polyvinyl alcohol for transdermal delivery of sumatriptan succinate. Drug Deliv. 2010;17:83–91.
Femenía-Font A, Padula C, Marra F, Balaguer-Fernández C, Merino V, López-Castellano A, et al. Bioadhesive monolayer film for the in vitro transdermal delivery of sumatriptan. J Pharm Sci. 2006;95:1561–9.
Rathore MS, Majumdar DK. Effect of formulation factors on in vitro transcorneal permeation of gatifloxacin from aqueous drops. AAPS PharmSciTech. 2006;7:E1–6.
Ubaidulla U, Reddy MVS, Ruckmani K, Ahmad FJ, Khar RK. Transdermal therapeutic system of carvedilol: effect of hydrophilic and hydrophobic matrix on in vitro and in vivo characteristics. AAPS PharmSciTech. 2007;8:2.
Aqil M, Ali A, Sultana Y, Najmi AK. Fabrication and evaluation of polymeric films for transdermal delivery of pinacidil. Pharmazie. 2004;59:631–5.
Okamoto N, Ito Y, Nagai N, Murao T, Takiguchi Y, Kurimoto T, et al. Preparation of ophthalmic formulations containing cilostazol as an anti-glaucoma agent and improvement in its permeability through the rabbit cornea. J Oleo Sci. 2010;59:423–30.
Srirangam R, Majumdar S. Passive asymmetric transport of hesperetin across isolated rabbit cornea. Int J Pharm. 2010;394:60–7.
Chandran S, Roy A, Saha RN. Effect of pH and formulation variables on in vitro transcorneal permeability of flurbiprofen: a technical note. AAPS PharmSciTech. 2008;9:1031–7.
Van Der Bijl P, Engelbrecht AH, Van Eyk AD, Meyer D. Comparative permeability of human and rabbit corneas to cyclosporin and tritiated water. J Ocul Pharmacol Ther. 2002;18:419–27.
Aburahma MH, Mahmoud AA. Biodegradable ocular inserts for sustained delivery of brimonidine tartarate: preparation and in vitro/in vivo evaluation. AAPS PharmSciTech. 2011;12:1335–47.
Franca JR, Foureaux G, Fuscaldi LL, et al. Bimatoprost-loaded ocular inserts as sustained release drug delivery systems for glaucoma treatment: in vitro and in vivo evaluation. PLoS One. 2014;9:e95461.
Jeganath S, Viji AA, Devi KS. Design and evaluation of controlled release ocuserts of indomethacin. Int J Pharm Sci Res. 2011;2:80–6.
Reddy DM, Reddy YK, Reddy DR, Kumar NV, Suresh M, Althaff M, et al. Formulation and evaluation of ciprofloxacin ocuserts. Res J Pharm Technol. 2011;4:1663–5.
Rao M, Nappinnai M, Raju S, Rao V, Reddy B. Fluconazole ocular inserts: formulation and in vitro evaluation. J Pharm Sci Res. 2010;2:344–50.
Kumari A, Sharma PK, Garg VK, Garg G. Ocular inserts—advancement in therapy of eye diseases. J Adv Pharm Technol Res. 2010;1:291–6.
Donnenfeld ED, Comstock TL, Proksch JW. Human aqueous humor concentrations of besifloxacin, moxifloxacin, and gatifloxacin after topical ocular application. J Cataract Refract Surg. 2011;37:1082–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest disclosure
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sebastián-Morelló, M., Calatayud-Pascual, M.A., Rodilla, V. et al. Ex vivo rabbit cornea diffusion studies with a soluble insert of moxifloxacin. Drug Deliv. and Transl. Res. 8, 132–139 (2018). https://doi.org/10.1007/s13346-017-0443-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-017-0443-y