Abstract
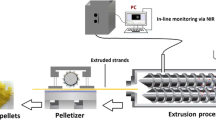
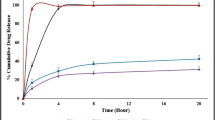
The purpose of this study was to explore poly(vinylpyrrolidone-co-vinyl acetate) (PVP VA64) as a novel release-modifier to tailor the drug release from ethylcellulose (EC)-based mini-matrices prepared via hot melt extrusion (HME). Quetiapine fumarate (QF) was selected as model drug. QF/EC/PVP VA64 mini-matrices were extruded with 30% drug loading. The physical state of QF in extruded mini-matrices was characterized using differential scanning calorimetry, X-ray powder diffraction, and confocal Raman microscopy. The release-controlled ability of PVP VA64 was investigated and compared with that of xanthan gum, crospovidone, and low-substituted hydroxypropylcellulose. The influences of PVP VA64 content and processing temperature on QF release behavior and mechanism were also studied. The results indicated QF dispersed as the crystalline state in all mini-matrices. The release of QF from EC was very slow as only 4% QF was released in 24 h. PVP VA64 exhibited the best ability to enhance the drug release as compared with other three release-modifiers. The drug release increased to 50–100% in 24 h with the addition of 20–40% PVP VA64. Increasing processing temperature slightly slowed down the drug release by decreasing free volume and pore size. The release kinetics showed good fit with the Ritger-Peppas model. The values of release exponent (n) increased as PVP VA64 is added (0.14 for pure EC, 0.41 for 20% PVP VA64, and 0.61 for 40% PVP VA64), revealing that the addition of PVP VA64 enhanced the erosion mechanism. This work presented a new polymer blend system of EC with PVP VA64 for sustained-release prepared via HME.












Similar content being viewed by others
References
Yang Y, Shen L, Li J, Shan WG. Preparation and evaluation of metoprolol tartrate sustained-release pellets using hot melt extrusion combined with hot melt coating. Drug Dev Ind Pharm. 2017;43:939–46.
Verstraete G, Van Renterghem J, Van Bockstal PJ, Kasmi S, De Geest BG, De Beer T, et al. Hydrophilic thermoplastic polyurethanes for the manufacturing of highly dosed oral sustained release matrices via hot melt extrusion and injection molding. Int J Pharm. 2016;506:214–21.
Albarahmieh E, Qi S, Craig DQM. Hot melt extruded transdermal films based on amorphous solid dispersions in Eudragit RS PO: the inclusion of hydrophilic additives to develop moisture-activated release systems. Int J Pharm. 2016;514:270–81.
Palem CR, Dudhipala NR, Battu SK, Repka MA, Yamsani MR. Development, optimization and in vivo characterization of domperidone-controlled release hot-melt-extruded films for buccal delivery. Drug Dev Ind Pharm. 2016;42:473–84.
Cosse A, Konig C, Lamprecht A, Wagner KG. Hot melt extrusion for sustained protein release: matrix erosion and in vitro release of PLGA-based implants. AAPS PharmSciTech. 2017;18:15–26.
Islam MT, Maniruzzaman M, Halsey SA, Chowdhry BZ, Douroumis D. Development of sustained-release formulations processed by hot-melt extrusion by using a quality-by-design approach. Drug Deliv Transl Res. 2014;4:377–87.
Verhoeven E, De Beer TR, Schacht E, Van den Mooter G, Remon JP, Vervaet C. Influence of polyethylene glycol/polyethylene oxide on the release characteristics of sustained-release ethylcellulose mini-matrices produced by hot-melt extrusion: in vitro and in vivo evaluations. Eur J Pharm Biopharm. 2009;72:463–70.
Verhoeven E, De Beer TR, Van den Mooter G, Remon JP, Vervaet C. Influence of formulation and process parameters on the release characteristics of ethylcellulose sustained-release mini-matrices produced by hot-melt extrusion. Eur J Pharm Biopharm. 2008;69:312–9.
Verhoeven E, Vervaet C, Remon JP. Xanthan gum to tailor drug release of sustained-release ethylcellulose mini-matrices prepared via hot-melt extrusion: in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2006;63:320–30.
De Brabander C, Vervaet C, Remon JP. Development and evaluation of sustained release mini-matrices prepared via hot melt extrusion. J Control Release. 2003;89:235–47.
Douroumis D. Hot-melt extrusion: pharmaceutical applications. United Kingdom: John Wiley & Sons; 1st edition. 2012.
Quinten T, Gonnissen Y, Adriaens E, De Beer T, Cnudde V, Masschaele B, et al. Development of injection moulded matrix tablets based on mixtures of ethylcellulose and low-substituted hydroxypropylcellulose. Eur J Pharm Sci. 2009;37:207–16.
Williams RO III, Watts AB, Miller DA. Formulating poorly water soluble drugs. 1st ed. New York: Springer; 2012.
Thiry J, Lebrun P, Vinassa C, Adam M, Netchacovitch L, Ziemons E, et al. Continuous production of itraconazole-based solid dispersions by hot melt extrusion: preformulation, optimization and design space determination. Int J Pharm. 2016;515:114–24.
Ashour EA. Majumdar S, Alsheteli A, Alshehri S, Alsulays B, Feng X, Gryczke Andreas, Kolter, K, Langley N, Repka MA. Hot melt extrusion as an approach to improve solubility, permeability and oral absorption of a psychoactive natural product, piperine. J Pharm Pharmacol. 2016;68:989–98.
Haser A, Huang SY, Listro T, White D, Zhang F. An approach for chemical stability during melt extrusion of a drug substance with a high melting point. Int J Pharm. 2017;524:55–64.
Kolter K, Karl M, Gryczke A. Hot-melt extrusion with BASF pharma polymers: extrusion compendium. BASF company. 2nd revised and enlarged edition. 2012.
Liu JP, Zhang F, McGinity JW. Properties of lipophilic matrix tablets containing phenylpropanolamine hydrochloride prepared by hot melt extrusion. Eur J Pharm Biopharm. 2001;52:181–90.
Crowley MM, Schroeder B, Fredersdorf A, Obara S, Talarico M, Kucera S, et al. Physicochemical properties and mechanism of drug release from ethyl cellulose matrix tablets prepared by direct compression and hot-melt extrusion. Int J Pharm. 2004;269:509–22.
Zhang YE, Tchao R, Schwartz JB. Effect of processing methods and heat treatment on the formation of wax matrix tablets for sustained drug release. Pharm Dev Technol. 2001;6:131–44.
Ritger PL, Peppas NA. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release. 1987;5:37–42.
Funding
The present work was financially supported by the National Sciences Funding of China (No. 51103184), Fundamental Research Funds for the Central Universities (No. 12ykpy08), and Medical Scientific Research Foundation of Guangdong Province (No. A2015169).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, Y., Lu, M. & Wu, C. PVP VA64 as a novel release-modifier for sustained-release mini-matrices prepared via hot melt extrusion. Drug Deliv. and Transl. Res. 8, 1670–1678 (2018). https://doi.org/10.1007/s13346-017-0437-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-017-0437-9