Abstract
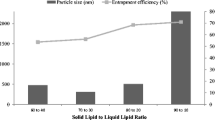
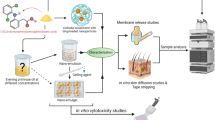
Lipid carrier-mediated transdermal drug delivery offers several advantages because it is non-irritating and non-toxic, provides effective control of drug release, and forms an adhesive film that hydrates the outer skin layers. However, to penetrate the deeper skin layers, these formulations need to overcome several barriers in the stratum corneum. This study evaluates factors influencing particle size and drug-loading capacity, which play a key role in drug permeation and efficacy. Diclofenac sodium was chosen as the model drug. The fabrication of diclofenac sodium-loaded lipid nanoparticles was optimized by modulating several parameters, including the lipids and surfactants employed, the drug/lipid ratio, and the pH of the aqueous phase. The physical properties and loading efficiencies of the nanoparticles were characterized. The optimized formulation was then dispersed into a polymer solution to form a gel, which demonstrated a sustained ex vivo permeation of diclofenac sodium over 24 h through excised rat skin and a higher drug penetrating capacity than that of a commercially available gel. In vivo anti-inflammatory activity was assessed in a rat carrageenan-induced paw edema model; the anti-edema effects of the prepared gel and commercially available gel over 24 h were comparable. The present findings indicate the effects of particle size and drug loading on the ability of nanostructured lipid carrier preparations to provide transdermal drug delivery.






Similar content being viewed by others
References
Arias JL, López-Viota M, López-Viota J, Delgado ÁV. Development of iron/ethylcellulose (core/shell) nanoparticles loaded with diclofenac sodium for arthritis treatment. Int J Pharm. 2009;382:270–6.
Liu D, Ge Y, Tang Y, Yuan Y, Zhang Q, Li R, Xu Q. Solid lipid nanoparticles for transdermal delivery of diclofenac sodium: preparation, characterization and in vitro studies. J Microencapsul. 2010;27:726–34.
Baraf HS, Fuentealba C, Greenwald M, Brzezicki J, O'Brien K, Soffer B, Polis A, Bird S, Kaur A, Curtis SP, EDGE Study Group. Gastrointestinal side effects of etoricoxib in patients with osteoarthritis: results of the Etoricoxib versus diclofenac sodium gastrointestinal tolerability and effectiveness (EDGE) trial. J Rheumatol. 2007;34:408–20.
Sintov AC, Botner S. Transdermal drug delivery using microemulsion and aqueous systems: influence of skin storage conditions on the in vitro permeability of diclofenac from aqueous vehicle systems. Int J Pharm. 2006;311:55–62.
Gaur PK, Purohit S, Kumar Y, Mishra S, Bhandari A. Preparation, characterization and permeation studies of a nanovesicular system containing diclofenac for transdermal delivery. Pharm Dev Technol. 2014;19:48–54.
Rehman K, Zulfakar MH. Recent advances in gel technologies for topical and transdermal drug delivery. Drug Dev Ind Pharm. 2014;40:433–40.
Schäfer-Korting M, Mehnert W, Korting H-C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv Drug Del Rev. 2007;59:427–43.
Cevc G, Vierl U. Nanotechnology and the transdermal route: a state of the art review and critical appraisal. J Control Release. 2010;141:277–99.
Vitorino C, Almeida J, Gonçalves LM, Almeida AJ, Sousa JJ, Pais AACC. Co-encapsulating nanostructured lipid carriers for transdermal application: from experimental design to the molecular detail. J Control Release. 2013;167:301–14.
Bhaskar K, Anbu J, Ravichandiran V, Venkateswarlu V, Rao YM. Lipid nanoparticles for transdermal delivery of flurbiprofen: formulation, in vitro, ex vivo and in vivo studies. Lipids Health Dis. 2009;8:1–15.
Alexander A, Dwivedi S, Ajazuddin, Giri TK, Saraf S, Saraf S, Tripathi DK. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J Control Release. 2012;164:26–40.
Shakeel F, Baboota S, Ahuja A, Ali J, Aqil M, Shafiq S. Nanoemulsions as vehicles for transdermal delivery of aceclofenac. AAPS PharmSciTech. 2007;8:191–9.
Kohli A, Alpar H. Potential use of nanoparticles for transcutaneous vaccine delivery: effect of particle size and charge. Int J Pharm. 2004;275:13–7.
Fang J-Y, Sung K, Lin H-H, Fang C-L. Transdermal iontophoretic delivery of diclofenac sodium from various polymer formulations: in vitro and in vivo studies. Int J Pharm. 1999;178:83–92.
Özgüney IS, Karasulu HY, Kantarci G, Sözer S, Güneri T, Ertan G. Transdermal delivery of diclofenac sodium through rat skin from various formulations. AAPS PharmSciTech. 2006;7:E39–45.
Teeranachaideekul V, Souto EB, Junyaprasert VB, Müller RH. Cetyl palmitate-based NLC for topical delivery of coenzyme Q10—development, physicochemical characterization and in vitro release studies. Eur J Pharm Biopharm. 2007;67:141–8.
Puglia C, Blasi P, Rizza L, Schoubben A, Bonina F, Rossi C, Ricci M. Lipid nanoparticles for prolonged topical delivery: an in vitro and in vivo investigation. Int J Pharm. 2008;357:295–304.
Vitorino C, Almeida A, Sousa J, Lamarche I, Gobin P, Marchand S, Couet W, Olivier JC, Pais A. Passive and active strategies for transdermal delivery using co-encapsulating nanostructured lipid carriers: in vitro vs. in vivo studies. Eur J Pharm Biopharm. 2014;86:133–44.
Pillai O, Panchagnula R. Transdermal delivery of insulin from poloxamer gel: ex vivo and in vivo skin permeation studies in rat using iontophoresis and chemical enhancers. J Control Release. 2003;89:127–40.
Gaur PK, Mishra S, Purohit S. Solid lipid nanoparticles of guggul lipid as drug carrier for transdermal drug delivery. Biomed Res Int. 2013;2013:750690.
Gaddam N, Aukunuru J. Systemic delivery of diclofenac sodium after topical application of gels incorporated with drug-loaded solid lipid nanoparticles (SLN). Asian J Pharm Res Health Care. 2010;2:177–87.
Tsai CC, Lin CC. Anti-inflammatory effects of Taiwan folk medicine ‘Teng-Khia-U’on carrageenan-and adjuvant-induced paw edema in rats. J Ethnopharmacol. 1998;64:85–9.
Jenning V, Gohla S. Comparison of wax and glyceride solid lipid nanoparticles (SLN®). Int J Pharm. 2000;196:219–22.
Tran TH, Ramasamy T, Cho HJ, Kim YI, Poudel BK, Choi HG, Yong CS, Kim JO. Formulation and optimization of raloxifene-loaded solid lipid nanoparticles to enhance oral bioavailability. J Nanosci Nanotechnol. 2014;14:4820–31.
Schubert M, Müller-Goymann C. Characterisation of surface-modified solid lipid nanoparticles (SLN): influence of lecithin and nonionic emulsifier. Eur J Pharm Biopharm. 2005;61:77–86.
Liu D, Jiang S, Shen H, Qin S, Liu J, Zhang Q, Li R, Xu Q. Diclofenac sodium-loaded solid lipid nanoparticles prepared by emulsion/solvent evaporation method. J Nanopart Res. 2011;13:2375–86.
Tran TH, Ramasamy T, Truong DH, Shin BS, Choi H-G, Yong CS, Yong CS, Kim JO. Development of vorinostat-loaded solid lipid nanoparticles to enhance pharmacokinetics and efficacy against multidrug-resistant cancer cells. Pharm Res. 2014;31:1978–88.
Tran TH, Ramasamy T, Truong DH, Choi H-G, Yong CS, Kim JO. Preparation and characterization of fenofibrate-loaded nanostructured lipid carriers for oral bioavailability enhancement. AAPS PharmSciTech. 2014;15:1509–15.
Souto E, Wissing S, Barbosa C, Müller R. Evaluation of the physical stability of SLN and NLC before and after incorporation into hydrogel formulations. Eur J Pharm Biopharm. 2004;58:83–90.
Cheong LWS, Heng PWS, Wong LF. Relationship between polymer viscosity and drug release from a matrix system. Pharm Res. 1992;9:1510–4.
Cevc G. Lipid vesicles and other colloids as drug carriers on the skin. Adv Drug Del Rev. 2004;56:675–711.
Zakrewsky M, Kumar S, Mitragotri S. Nucleic acid delivery into skin for the treatment of skin disease: proofs-of-concept, potential impact, and remaining challenges. J Control Release. 2015;219:445–56.
Dragicevic N, Maibach HI. Percutaneous penetration enhancers chemical methods in penetration enhancement: drug manipulation strategies and vehicle effects. 2015th ed. Berlin: Springer; 2015.
Kirjavainen M, Mönkkönen J, Saukkosaari M, Valjakka-Koskela R, Kiesvaara J, Urtti A. Phospholipids affect stratum corneum lipid bilayer fluidity and drug partitioning into the bilayers. J Control Release. 1999;58:207–14.
Acknowledgements
We would like to acknowledge the Ethics Committee of Medicine and Pharmacy Research, Military Medical University (Hanoi, Vietnam) for approving our study protocol.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this paper.
Animal studies
All institutional and national guidelines for the care and use of laboratory animals were followed.
Rights and permissions
About this article
Cite this article
Nguyen, C.N., Nguyen, T.T.T., Nguyen, H.T. et al. Nanostructured lipid carriers to enhance transdermal delivery and efficacy of diclofenac. Drug Deliv. and Transl. Res. 7, 664–673 (2017). https://doi.org/10.1007/s13346-017-0415-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-017-0415-2