Abstract
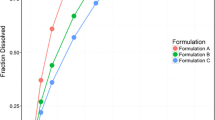
Elacridar is an inhibitor of the permeability glycoprotein (P-gp) and the breast cancer resistance protein (BCRP) and is a promising absorption enhancer of drugs that are substrates of these drug-efflux transporters. However, elacridar is practically insoluble in water, resulting in low bioavailability which currently limits its clinical application. We evaluated the in vitro dissolution and clinical pharmacokinetics of a novel amorphous solid dispersion (ASD) tablet containing elacridar. The dissolution from ASD tablets was compared to that from a crystalline powder mixture in a USP type II dissolution apparatus. The pharmacokinetics of the ASD tablet were evaluated in an exploratory clinical study at oral doses of 25, 250, or 1000 mg in 12 healthy volunteers. A target Cmax was set at ≥ 200 ng/mL based on previous clinical data. The in vitro dissolution from the ASD tablet was 16.9 ± 3.7 times higher compared to that from a crystalline powder mixture. Cmax and AUC0-∞ increased linearly with dose over the explored range. The target Cmax of ≥ 200 ng/mL was achieved at the 1000-mg dose level. At this dose, the Cmax and AUC0-∞ were 326 ± 67 ng/mL and 13.4 ± 8.6 · 103 ng · h/mL, respectively. In summary, the ASD tablet was well tolerated, resulted in relevant pharmacokinetic exposure, and can be used for proof-of-concept clinical studies.



Similar content being viewed by others
Abbreviations
- ABCB1:
-
ATP Binding Cassette B1
- ABCG2:
-
ATP Binding Cassette G2
- ASD:
-
Amorphous solid dispersion
- BCRP:
-
Breast cancer resistance protein
- BCS:
-
Biopharmaceutics Classification System
- CNS:
-
Central nervous system
- DMSO:
-
Dimethyl sulfoxide
- GMP:
-
Good manufacturing practices
- P-gp:
-
Permeability glycoprotein
- PVPK30:
-
Povidone K30
- SDS:
-
Sodium dodecyl sulfate
References
Shukla S, Ohnuma S, Ambudkar SV. Improving cancer chemotherapy with modulators of ABC drug transporters. Curr Drug Targets. 2011;12:621–30.
van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015;19:1–12.
Hyafil F, Vergely C, Du Vignaud P, Grand-Perret T. In vitro and in vivo reversal of multidrug resistance by in vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 1993;53:4595–602.
de Bruin M, Miyake K, Litman T, Robey R, Bates SE. Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter. MXR Cancer Lett. 1999;146:117–26.
Malingré MM, Beijnen JH, Rosing H, Koopman FJ, Jewell RC, Paul EM, et al. Co-administration of GF120918 significantly increases the systemic exposure to oral paclitaxel in cancer patients. Br J Cancer. 2001;84:42–7.
Kuppens IE, Witteveen EO, Jewell RC, Radema SA, Paul EM, Mangum SG, et al. A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (GF120918) and oral topotecan in cancer patients. Clin Cancer Res. 2007;13:3276–85.
Kruijtzer CMF, Beijnen JH, Rosing H, Ten Bokkel Huinink WW, Schot M, Jewell RC, et al. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J Clin Oncol. 2002;20:2943–50.
Sane R, Mittapalli RK, Elmquist WF. Development and evaluation of a novel microemulsion formulation of elacridar to improve its bioavailability. J Pharm Sci. 2013;102:1343–54.
Bunt AMG, van Tellingen O. Efflux inhibitor compositions and methods of treatment use the same, Patent number US 2014/0235631 A1. p. 1–33.
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system guidance for industry. May 2015, Revision 1; Available from: http://www.fda.gov/downloads/Drugs/GuidanceCompliance RegulatoryInformation/Guidances/ucm070246.pdf. Accessed 15 March 2016.
Ward KW, Azzarano LM. Preclinical pharmacokinetic properties of the P-glycoprotein inhibitor GF120918A (HCl salt of GF120918, 9,10-dihydro-5-methoxy-9-oxo-N-[4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]phenyl]-4-acridine-carboxamide) in the mouse, rat,dog, and monkey. J Pharmacol Exp Ther. 2004;310:703–9.
Planting AST, Sonneveld P, van der Gaast A, Sparreboom A, van der Burg MEL, Luyten GPM, et al. A phase I and pharmacologic study of the MDR converter GF120918 in combination with doxorubicin in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2005;55:91–9.
Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm. 2011;420:1–10.
van Hoogevest P, Liu X, Fahr A. Drug delivery strategies for poorly water-soluble drugs: the industrial perspective. Expert Opin. Drug Deliv. 2011;8:1481–500.
Chiou WL, Riegelman S. Pharmaceutical applications of solid dispersion systems. J Pharm Sci. 1971;60:1281–302.
Janssens S, van den Mooter G. Review: physical chemistry of solid dispersions. J Pharm Pharmacol. 2009;61:1571–86.
Alam MA, Ali R, Al-Jenoobi FI, Al-Mohizea AM. Solid dispersions: a strategy for poorly aqueous soluble drugs and technology updates. Expert Opin Drug Deliv. 2012;9:1419–40.
He Y, Ho C. Amorphous solid dispersions: utilization and challenges in drug discovery and development. J Pharm Sci. 2015;104:3237–58.
Sharp MJ, Mader CJ, Strachan C. Synthesis of acridine derivative multidrug-resistant inhibitors, International Patent: WO1998052923 A1. 1998. p. 1–21.
USP. United States Pharmacopoeia National Formulary Online. 2015; Available from: www.usp.org/usp-nf.
den Brok MWJ, Nuijen B, Lutz C, Opitz HG, Beijnen JH. Pharmaceutical development of a lyophilised dosage form for the investigational anticancer agent Imexon using dimethyl sulfoxide as solubilising and stabilising agent. J Pharm Sci. 2005;94:1101–14.
PhEur. European Pharmacopoeia Online. 2016; Available from: http://online.edqm.eu
Sawicki E, Hillebrand MJ, Rosing H, Schellens JHM, Nuijen B, Beijnen JH. Validation of a liquid chromatographic method for the pharmaceutical quality control of products containing elacridar. J Pharm Anal. 2016;6:268–75.
Stokvis E, Rosing H, Causon RC, Schellens JHM, Beijnen JH. Quantitative analysis of the P-glycoprotein inhibitor elacridar (GF120918) in human and dog plasma using liquid chromatography with tandem mass spectrometric detection. J Mass Spectrom. 2004;39:1122–30.
Sun DD, Lee PI. Haste makes waste: the interplay between dissolution and precipitation of supersaturating formulations. AAPS J. 2015;17:1317–26.
Patel DD, Anderson BD. Maintenance of supersaturation II: indomethacin crystal growth kinetics versus degree of supersaturation. J Pharm Sci. 2013;102:1544–53.
Brouwers J, Brewster ME, Augustijns P. Supersaturating drug delivery systems: the answer to solubility-limited oral bioavailability? J Pharm Sci. 2009;98:2549–472.
Padowski JM, Pollack GM. Examination of the ability of the nasal administration route to confer a brain exposure advantage for three chemical inhibitors of P-glycoprotein. J Pharm Sci. 2010;99:3226–33.
Acknowledgments
Funding for this research was provided by a personal grant from The Netherlands Cancer Institute to Dr. N. Steeghs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiments comply with the current laws of the country in which they were performed.
All procedures were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all human volunteers for being included in the study.
Conflict of interest
Olaf van Tellingen is co-inventor of a patent application (Bunt and Van Tellingen, 2014; US 20140235631A1) dealing with development of an improved oral formulation for elacridar. All other authors declare they have no conflict of interest.
Funding
Funds for this research were provided by a personal grant from The Netherlands Cancer Institute to Dr. N. Steeghs.
Rights and permissions
About this article
Cite this article
Sawicki, E., Verheijen, R.B., Huitema, A.D. et al. Clinical pharmacokinetics of an amorphous solid dispersion tablet of elacridar. Drug Deliv. and Transl. Res. 7, 125–131 (2017). https://doi.org/10.1007/s13346-016-0346-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-016-0346-3