Abstract
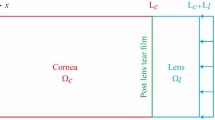
Currently, most in vitro drug release studies for ophthalmic applications are carried out in static sink conditions. Although this procedure is simple and useful to make comparative studies, it does not describe adequately the drug release kinetics in the eye, considering the small tear volume and flow rates found in vivo. In this work, a microfluidic cell was designed and used to mimic the continuous, volumetric flow rate of tear fluid and its low volume. The suitable operation of the cell, in terms of uniformity and symmetry of flux, was proved using a numerical model based in the Navier-Stokes and continuity equations. The release profile of a model system (a hydroxyethyl methacrylate-based hydrogel (HEMA/PVP) for soft contact lenses (SCLs) loaded with diclofenac) obtained with the microfluidic cell was compared with that obtained in static conditions, showing that the kinetics of release in dynamic conditions is slower. The application of the numerical model demonstrated that the designed cell can be used to simulate the drug release in the whole range of the human eye tear film volume and allowed to estimate the drug concentration in the volume of liquid in direct contact with the hydrogel. The knowledge of this concentration, which is significantly different from that measured in the experimental tests during the first hours of release, is critical to predict the toxicity of the drug release system and its in vivo efficacy. In conclusion, the use of the microfluidic cell in conjunction with the numerical model shall be a valuable tool to design and optimize new therapeutic drug-loaded SCLs.





Similar content being viewed by others
Notes
The term sink conditions is usually defined as the volume of medium at least greater than three times that required to form a saturated solution of a drug substance.
References
Gulsen D, Li C-C, Chauhan A. Dispersion of DMPC liposomes in contact lenses for ophthalmic drug delivery. Curr Eye Res. 2005;30(12):1071–80.
Nguyen D, Hui A, Weeks A, Heynen M, Joyce E, Sheardown H, et al. Release of ciprofloxacin-HCl and dexamethasone phosphate by hyaluronic acid containing silicone polymers. Materials. 2012;5(4):684–98.
Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348–60.
Ali M, Horikawa S, Venkatesh S, Saha J, Hong JW, Byrne ME. Zero-order therapeutic release from imprinted hydrogel contact lenses within in vitro physiological ocular tear flow. J Control Release. 2007;124(3):154–62.
ElShaer A, Ghatora B, Mustafa S, Raid A. Contact lenses as drug reservoirs & delivery systems: the successes & challenges. Ther Deliv. 2014;5(10):1085–100.
Salminen L. Review: systemic absorption of topically applied ocular drugs in humans. J Ocul Pharmacol. 1990;6(3):243–9.
Bengani LC, Chauhan A. Extended delivery of an anionic drug by contact lens loaded with a cationic surfactant. Biomaterials. 2013;34(11):2814–21.
Bourlais CL, Acar L, Zia H, Sado P, Needham T, Leverge R. Ophthalmic drug delivery systems—recent advances. Prog Retin Eye Res. 1998;17(1):33–58.
Kuno N, Fujii S. Recent advances in ocular drug delivery systems. Polymers (Basel). 2011;3(1):193–221.
Nichols JJ. Contact lenses 2014. vol. 30, Contact lens spectrum. 2015. 22–7.
Rubinstein MP. Applications of contact lens devices in the management of corneal disease. Eye. 2003;17(8):872–6.
Hehl EM, Beck R, Luthard K, Guthoff R, Drewelow B. Improved penetration of aminoglycosides and fluorozuinolones into the aqueous humour of patients by means of Acuvue contact lenses. Eur J Clin Pharmacol. 1999;55(4):317–23.
Kakisu K, Matsunaga T, Kobayakawa S, Sato T, Tochikubo T. Development and efficacy of a drug-releasing soft contact lens. Invest Ophthalmol Vis Sci. 2013;54(4):2551–61.
Ciolino JB, Hoare TR, Iwata NG, Behlau I, Dohlman CH, Langer R, et al. A drug-eluting contact lens. Invest Ophthalmol Vis Sci. 2009;50(7):3346–52.
Nicolson PC, Vogt J. Soft contact lens polymers: an evolution. Biomaterials. 2001;22(24):3273–83.
Yañez F, Martikainen L, Braga MEM, Alvarez-Lorenzo C, Concheiro A, Duarte CMM, et al. Supercritical fluid-assisted preparation of imprinted contact lenses for drug delivery. Acta Biomater. 2011;7(3):1019–30.
dos Santos J-FR, Alvarez-Lorenzo C, Silva M, Balsa L, Couceiro J, Torres-Labandeira J-J, et al. Soft contact lenses functionalized with pendant cyclodextrins for controlled drug delivery. Biomaterials. 2009;30(7):1348–55.
Alvarez-Lorenzo C, Yañez F, Barreiro-Iglesias R, Concheiro A. Imprinted soft contact lenses as norfloxacin delivery systems. J Control Release. 2006;113(3):236–44.
Gulsen D. Ophthalmic drug delivery through contact lenses. Invest Ophthalmol Vis Sci. 2004;45(7):2342–7.
Tieppo A, Boggs AC, Pourjavad P, Byrne ME. Analysis of release kinetics of ocular therapeutics from drug releasing contact lenses: best methods and practices to advance the field. Cont Lens Anterior Eye. 2014;37(4):305–13.
White CJ, Tieppo A, Byrne ME. Controlled drug release from contact lenses: a comprehensive review from 1965-present. J Drug Deliv Sci Technol. 2011;21(5):368–84.
Mishima S, Gasset A, Klyce SD, Baum JL. Determination of tear volume and tear flow. Invest Ophthalmol Vis Sci. 1966;5:264–76.
Robert L. Stamper, Marc F. Lieberman MVD. Becker-Shaffer’s diagnosis and therapy of the glaucomas. In: Elsevier M, editor. Part 5, 8th Edition.
Linsy Farris R. Tear analysis in contact lens wearers. Trans Am Ophthalmol Soc. 1985;83:501–45.
Glasson MJ, Stapleton F, Keay L, Willcox MDP. The effect of short term contact lens wear on the tear film and ocular surface characteristics of tolerant and intolerant wearers. Cont Lens Anterior Eye. 2006;29(1):41–7.
Tieppo A, Pate KM, Byrne ME. In vitro controlled release of an anti-inflammatory from daily disposable therapeutic contact lenses under physiological ocular tear flow. Eur J Pharm Biopharm. 2012;81(1):170–7.
Bajgrowicz M, Phan C-M, Subbaraman L, Jones L. Release of ciprofloxacin and moxifloxacin from daily disposable contact lenses from an in vitro eye model. Invest Ophthalmol Vis Sci. 2015;56(4):2234–42.
Vazquez R, Nogueira R, Orfão M, Mata JL, Saramago B. Stability of triglyceride liquid films on hydrophilic and hydrophobic glasses. J Colloid Interface Sci. 2006;299(1):274–82.
Pishko GL, Lee S, Wanakule P, Sarntinoranont M. Hydraulic permeability of a hydrogel-based contact lens membrane for low flow rates. J Appl Polym Sci. 2007;104:3730–5.
Refojo MF. Permeation of water through some hydrogels. J Appl Polym Sci. 1965;9(10):3417–26.
Diclofenac sodium salt, material safety DATA, product informations by Cayman Chemical Company. 2012.
German EJ, Hurst MA, Wood D. Reliability of drop size from multi-dose eye drop bottles: is it cause for concern? Eye. 1999;13:93–100.
Acknowledgments
Acknowledgements are due to Fundação para a Ciência e a Tecnologia for funding through the projects PEst-OE/QUI/UI0100/2013 and M-ERA.NET/0005/2012 and support, through IDMEC, under LAETA, of project UID/EMS/50022/2013. A.F.R. Pimenta acknowledges Fundação para a Ciência e a Tecnologia for the PhD Grant SFRH/BD/52334/2013. The authors also thank Professor J.R. Ascenso (CQE-IST, University of Lisbon) for the NMR measurement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Pimenta, A.F.R., Valente, A., Pereira, J.M.C. et al. Simulation of the hydrodynamic conditions of the eye to better reproduce the drug release from hydrogel contact lenses: experiments and modeling. Drug Deliv. and Transl. Res. 6, 755–762 (2016). https://doi.org/10.1007/s13346-016-0303-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-016-0303-1