Abstract
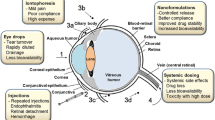
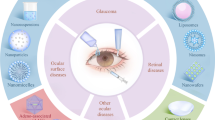
The complexity of the structure and nature of the eye emanates a challenge for drug delivery to formulation scientists. Lower bioavailability concern of conventional ocular formulation provokes the interest of researchers in the development of novel drug delivery system. Nanotechnology-based formulations have been extensively investigated and found propitious in improving bioavailability of drugs by overcoming ocular barriers prevailing in the eye. The advent of nanocrystals helped in combating the problem of poorly soluble drugs specifically for oral and parenteral drug delivery and led to development of various marketed products. Nanocrystal-based formulations explored for ocular drug delivery have been found successful in achieving increase in retention time, bioavailability, and permeability of drugs across the corneal and conjunctival epithelium. In this review, we have highlighted the ocular physiology and barriers in drug delivery. A comparative analysis of various nanotechnology-based ocular formulations is done with their pros and cons. Consideration is also given to various methods of preparation of nanocrystals with their patented technology. This article highlights the success achieved in conquering various challenges of ocular delivery by the use of nanocrystals while emphasizing on its advantages and application for ocular formulation. The perspectives of nanocrystals as an emerging flipside to explore the frontiers of ocular drug delivery are discussed.



Similar content being viewed by others
References
Gan L, Wang J, Jiang M, Bartlett H, Ouyang D, Eperjesi F, et al. Recent advances in topical ophthalmic drug delivery with lipid-based nanocarriers. Drug Discov Today. 2013;18(5):290–7.
Conway BR. Recent patents on ocular drug delivery systems. Recent Pat Drug Deliv Formul. 2008;2(1):1–8.
Araújo J, Gonzalez E, Egea MA, Garcia ML, Souto EB. Nanomedicines for ocular NSAIDs: safety on drug delivery. Nanomedicine. 2009;5(4):394–401.
Yang X, Trinh HM, Agrahari V, Sheng Y, Pal D, Mitra AK. Nanoparticle-based topical ophthalmic gel formulation for sustained release of hydrocortisone butyrate. AAPS PharmSciTech. 2015:1–13
Honda M, Asai T, Oku N, Araki Y, Tanaka M, Ebihara N. Liposomes and nanotechnology in drug development: focus on ocular targets. Int J Nanomedicine. 2013;8:495–504.
Gaudana R, Jwala J, Boddu SH, Mitra AK. Recent perspectives in ocular drug delivery. Pharm Res. 2009;26(5):1197–216.
Del Amo EM, Urtti A. Current and future ophthalmic drug delivery systems: a shift to the posterior segment. Drug Discov Today. 2008;13(3):135–43.
Lang JC. Ocular drug delivery conventional ocular formulations. Adv Drug Deliv Rev. 1995;16(1):39–43.
Achouri D, Alhanout K, Piccerelle P, Andrieu V. Recent advances in ocular drug delivery. Drug Dev Ind Pharm. 2013;39(11):1599–617.
Kaur IP, Smitha R. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev Ind Pharm. 2002;28(4):353–69.
Prabhu P, Dubey A, Parth V, Ghate V. Investigation of hydrogel membranes containing combination of gentamicin and dexamethasone for ocular delivery. Int J Pharm Investig. 2015;5(4):214–25.
Meisner D, Mezei M. Liposome ocular delivery systems. Adv Drug Deliv Rev. 1995;16(1):75–93.
Abdelbary G, El-gendy N. Niosome-encapsulated gentamicin for ophthalmic controlled delivery. AAPS PharmSciTech. 2008;9(3):740–7.
Vandamme TF. Microemulsions as ocular drug delivery systems: recent developments and future challenges. Prog Retin Eye Res. 2002;21(1):15–34.
Kambhampati SP, Kannan RM. Dendrimer nanoparticles for ocular drug delivery. J Ocul Pharmacol Ther. 2013;29(2):151–65.
Barar I, Omidi Y. Nanoparticles for ocular drug delivery. Nanomedicine Drug Deliv. 2013;287.
Jiao J. Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv Drug Deliv Rev. 2008;60(15):1663–73.
Seyfoddin A, Shaw J, Al-Kassas R. Solid lipid nanoparticles for ocular drug delivery. Drug Deliv. 2010;17(7):467–89.
Popov A, Enlow EM, Bourassa J, Gardner CR, Chen H, Ensign LM et al. Inventors; The Johns Hopkins University, assignee. Nanocrystals, compositions, and methods that aid particle transport in mucus. Baltimore, MD. US 9056057. 2013.
Tuomela A, Liu P, Puranen J, Rönkkö S, Laaksonen T, Kalesnykas G, et al. Brinzolamide nanocrystal formulations for ophthalmic delivery: reduction of elevated intraocular pressure in vivo. Int J Pharm. 2014;467(1):34–41.
Reimondez-Troitiño S, Csaba N, Alonso M, de la Fuente M. Nanotherapies for the treatment of ocular diseases. Eur J Pharm Biopharm. 2015;95:279–93.
Holly FJ. Formation and stability of the tear film. Int Ophthalmol Clin. 1973;13(1):73–96.
Gunda S, Hariharan S, Mitra AK. Corneal absorption and anterior chamber pharmacokinetics of dipeptide monoester prodrugs of ganciclovir (GCV): in vivo comparative evaluation of these prodrugs with Val-GCV and GCV in rabbits. J Ocul Pharmacol Ther. 2006;22(6):465–76.
Grass GM, Robinson JR. Mechanisms of corneal drug penetration I: in vivo and in vitro kinetics. J Pharm Sci. 1988;77(1):3–14.
K-i H, Lee VH, Kim K-J. Roles of the conjunctiva in ocular drug delivery: a review of conjunctival transport mechanisms and their regulation. Eur J Pharm Biopharm. 2005;60(2):227–40.
Kim YC, Oh KH, Edelhauser HF, Prausnitz MR. Formulation to target delivery to the Ciliary body and choroid via the suprachoroidal space of the eye using microneedles. Eur J Pharm Biopharm. 2015;95:398–406.
Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open ophthalmol J. 2010;4:52–9.
Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58(11):1131–5.
Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348–60.
Sahoo SK, Dilnawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov Today. 2008;13(3):144–51.
Baeyens V, Gurny R. Chemical and physical parameters of tears relevant for the design of ocular drug delivery formulations. Pharm Acta Helv. 1997;72(4):191–202.
Coursey TG, Henriksson JT, Marcano DC, Shin CS, Isenhart LC, Ahmad F, et al. Dexamethasone nanowafer as an effective therapy for dry eye disease. J Control Release. 2015;213:168–74.
Bron A, Tiffany J, Gouveia S, Yokoi N, Voon L. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78(3):347–60.
Ranta V-P, Mannermaa E, Lummepuro K, Subrizi A, Laukkanen A, Antopolsky M, et al. Barrier analysis of periocular drug delivery to the posterior segment. J Control Release. 2010;148(1):42–8.
Ye T, Yuan K, Zhang W, Song S, Chen F, Yang X, et al. Prodrugs incorporated into nanotechnology-based drug delivery systems for possible improvement in bioavailability of ocular drugs delivery. Asian J Pharm Sci. 2013;8(4):207–17.
X-j Y, Wang Y, Fu-Shin XY. Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Invest Ophthalmol Vis Sci. 2000;41(13):4093–100.
Toda R, Kawazu K, Oyabu M, Miyazaki T, Kiuchi Y. Comparison of drug permeabilities across the blood–retinal barrier, blood–aqueous humor barrier, and blood–brain barrier. J Pharm Sci. 2011;100(9):3904–11.
Raviola G. The structural basis of the blood-ocular barriers. Exp Eye Res. 1977;25:27–63.
Cunha-Vaz J. The blood-retinal barriers. Doc Ophthalmol. 1976;41(2):287–327.
Khurana V, Patel SP, Agrahari V, Pal D, Mitra AK. Novel pentablock copolymer based nanoparticles containing pazopanib: a potential therapy for ocular neovascularization. Recent Pat Nanomedicine. 2014;4(1):57–68.
Almazan A, Lee SS, Ross AD, Robinson MR. 7 Barriers to transscleral drug delivery to the retina. Ocular Drug Delivery Systems: Barriers and Application of Nanoparticulate Systems. 2012;133.
Gaudana R, Barot M, Patel A, Khurana V, Mitra AK. Barriers for posterior segment ocular drug delivery. In: Mitra AK, editor. Treatise on Ocular Drug Delivery. Bentham E Books, 2013;68–95.
Occhiutto ML, Freitas FR, Maranhao RC, Costa VP. Breakdown of the blood-ocular barrier as a strategy for the systemic use of nanosystems. Pharmaceutics. 2012;4(2):252–75.
Pijls RT, Cruysberg LP, Nuijts RM, Dias AA, Koole LH. Capacity and tolerance of a new device for ocular drug delivery. Int J Pharm. 2007;341(1):152–61.
Fangueiro J, Veiga F, Silva A, Souto E. Ocular drug delivery-new strategies for targeting anterior and posterior segments of the eye. Curr Pharm Des. 2016;22(9):1135–46.
Suresh PK, Sah AK. Nanocarriers for ocular delivery for possible benefits in the treatment of anterior uveitis: focus on current paradigms and future directions. Expert Opin Drug Deliv. 2014;11(11):1747–68.
Sakurai E, Ozeki H, Kunou N, Ogura Y. Effect of particle size of polymeric nanospheres on intravitreal kinetics. Ophthalmic Res. 2001;33(1):31–6.
Bourges J-L, Gautier SE, Delie F, Bejjani RA, Jeanny J-C, Gurny R, et al. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Invest Ophthalmol Vis Sci. 2003;44(8):3562–9.
Dilnawaz F, Sahoo SK. Nanotechnology-based ophthalmic drug delivery system. Focal controlled drug delivery. Springer; 2014. p. 225–41.
Diebold Y, Jarrín M, Saez V, Carvalho EL, Orea M, Calonge M, et al. Ocular drug delivery by liposome–chitosan nanoparticle complexes (LCS-NP). Biomaterials. 2007;28(8):1553–64.
Radomska-Soukharev A, Wojciechowska J. Microemulsions as potential ocular drug delivery systems: phase diagrams and physical properties depending on ingredients. Acta Pol Pharm. 2005;62(6):465–71.
Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45(1):89–121.
Jiang J, Moore JS, Edelhauser HF, Prausnitz MR. Intrascleral drug delivery to the eye using hollow microneedles. Pharm Res. 2009;26(2):395–403.
Bariya SH, Gohel MC, Mehta TA, Sharma OP. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64(1):11–29.
Abdelkader H, Wu Z, Al-Kassas R, Alany RG. Niosomes and discomes for ocular delivery of naltrexone hydrochloride: morphological, rheological, spreading properties and photo-protective effects. Int J Pharm. 2012;433(1):142–8.
Yavuz B, Bozdağ Pehlivan S, Ünlü N. Dendrimeric systems and their applications in ocular drug delivery. Sci World J. 2013;2013:1–13.
Christie JG, Kompella UB. Ophthalmic light sensitive nanocarrier systems. Drug Discov Today. 2008;13(3):124–34.
Sahoo SK, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug Discov Today. 2003;8(24):1112–20.
Katzer T, Chaves P, Bernardi A, Pohlmann A, Guterres SS, Ruver Beck RC. Prednisolone-loaded nanocapsules as ocular drug delivery system: development, in vitro drug release and eye toxicity. J Microencapsul. 2014;31(6):519–28.
Vasir JK, Tambwekar K, Garg S. Bioadhesive microspheres as a controlled drug delivery system. Int J Pharm. 2003;255(1):13–32.
Patravale V, Kulkarni R. Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol. 2004;56(7):827–40.
Malkani A, Date AA, Hegde D. Celecoxib nanosuspension: single-step fabrication using a modified nanoprecipitation method and in vivo evaluation. Drug Deliv Transl Res. 2014;4(4):365–76.
Shelar DB, Pawar SK, Vavia PR. Fabrication of isradipine nanosuspension by anti-solvent microprecipitation–high-pressure homogenization method for enhancing dissolution rate and oral bioavailability. Drug Deliv Transl Res. 2013;3(5):384–91.
Junyaprasert VB, Morakul B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J Pharm Sci. 2015;10(1):13–23.
Chen H, Khemtong C, Yang X, Chang X, Gao J. Nanonization strategies for poorly water-soluble drugs. Drug Discov Today. 2011;16(7):354–60.
Junghanns J-UA, Müller RH. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomedicine. 2008;3(3):295–310.
Möschwitzer JP. Drug nanocrystals in the commercial pharmaceutical development process. Int J Pharm. 2013;453(1):142–56.
Sun B, Yeo Y. Nanocrystals for the parenteral delivery of poorly water-soluble drugs. Curr Opin Solid State Mater Sci. 2012;16(6):295–301.
Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3(9):785–96.
Keck CM, Müller RH. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur J Pharm Biopharm. 2006;62(1):3–16.
Gulsun T, Gursoy R, Levent O. Nanocrystal technology for oral delivery of poorly water soluble drugs. FARAD J Pharm Sci. 2009;34:55–65.
Shah T, Patel D, Hirani J, Amin A. Nanosuspensions as a drug delivery system: a comprehensive review. Drug Deliv Technol. 2007;7(9):42–52.
Merisko-Liversidge E, Liversidge GG. Nanosizing for oral and parenteral drug delivery: a perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv Drug Deliv Rev. 2011;63(6):427–40.
Sinha B, Müller RH, Möschwitzer JP. Bottom-up approaches for preparing drug nanocrystals: formulations and factors affecting particle size. Int J Pharm. 2013;453(1):126–41.
de Waard H, Frijlink HW, Hinrichs WL. Bottom-up preparation techniques for nanocrystals of lipophilic drugs. Pharm Res. 2011;28(5):1220–3.
Chin WWL, Parmentier J, Widzinski M, Tan EH, Gokhale R. A brief literature and patent review of nanosuspensions to a final drug product. J Pharm Sci. 2014;103(10):2980–99.
Gadad A, Kumar SV, Dandagi P, Bolmol U, Pallavi NP. Nanoparticles and their therapeutic applications in pharmacy. Int J Pharm Sci Nanotechnol. 2014;7(3):2509–19.
Che E, Zheng X, Sun C, Chang D, Jiang T, Wang S. Drug nanocrystals: a state of the art formulation strategy for preparing the poorly water-soluble drugs. Asian J Pharm Sci. 2012;7(2):85–95.
Keck C. Nanocrystals and amorphous nanoparticles and method for production of the same by a low energy process. US 9040105. 2012.
Müller RH, Gohla S, Keck CM. State of the art of nanocrystals–special features, production, nanotoxicology aspects and intracellular delivery. Eur J Pharm Biopharm. 2011;78(1):1–9.
Petersen R. inventor Nanocrystals for use in topical cosmetic formulations and method of production thereof patent US 20100047297. 2010.
Haynes DH. inventor Phospholipid-coated microcrystals: injectable formulations of water-insoluble drugs. US 5091188. 1992.
Scholz P, Arntjen A, Müller RH, Keck CM. ARTcrystal® process for industrial nanocrystal production—optimization of the ART MICCRA pre-milling step. Int J Pharm. 2014;465(1):388–95.
Liversidge GG, Cundy KC, Bishop JF, Czekai DA. inventors; Sterling Drug Inc., assignee. Surface modified drug nanoparticles. New York. US 5145684, 1992.
Srivalli KMR, Mishra B. Drug nanocrystals: a way toward scale-up. Saudi Pharm J. 2014:1–19
Gao L, Zhang D, Chen M. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J Nanoparticle Res. 2008;10(5):845–62.
Krause K, Müller R. Production and characterisation of highly concentrated nanosuspensions by high pressure homogenisation. Int J Pharm. 2001;214(1):21–4.
Shegokar R, Müller RH. Nanocrystals: industrially feasible multifunctional formulation technology for poorly soluble actives. Int J Pharm. 2010;399(1):129–39.
Baba K, Tanaka Y, Kubota A, Kasai H, Yokokura S, Nakanishi H, et al. A method for enhancing the ocular penetration of eye drops using nanoparticles of hydrolyzable dye. J Control Release. 2011;153(3):278–87.
Ali HS, York P, Ali AM, Blagden N. Hydrocortisone nanosuspensions for ophthalmic delivery: a comparative study between microfluidic nanoprecipitation and wet milling. J Control Release. 2011;149(2):175–81.
Kassem M, Rahman AA, Ghorab M, Ahmed M, Khalil R. Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int J Pharm. 2007;340(1):126–33.
Baba K, Nishida K. Steroid nanocrystals prepared using the nano spray dryer B-90. Pharmaceutics. 2013;5(1):107–14.
Gupta S, Samanta MK, Raichur AM. Dual-drug delivery system based on in situ gel-forming nanosuspension of forskolin to enhance antiglaucoma efficacy. AAPS PharmSciTech. 2010;11(1):322–35.
Nagai N, Yoshioka C, Mano Y, Tnabe W, Ito Y, Okamoto N, et al. A nanoparticle formulation of disulfiram prolongs corneal residence time of the drug and reduces intraocular pressure. Exp Eye Res. 2015;132:115–23.
Nagai N, Ono H, Hashino M, Ito Y, Okamoto N, Shimomura Y. Improved corneal toxicity and permeability of tranilast by the preparation of ophthalmic formulations containing its nanoparticles. J Oleo Sci. 2014;63(2):177–86.
Kim JH, Jang SW, Han SD, Hwang HD, Choi H-G. Development of a novel ophthalmic ciclosporin A-loaded nanosuspension using top-down media milling methods. Pharmazie. 2011;66(7):491–5.
Schopf L, Enlow E, Popov A, Bourassa J, Chen H. Ocular pharmacokinetics of a novel loteprednol etabonate 0.4% ophthalmic formulation. Ophthalmol Ther. 2014;3(1–2):63–72.
Baba K, Nishida K, Hashida N, inventors; Osaka University, assignee. Method for producing an aqueous dispersion of drug nanoparticles and use thereof. Osaka, US 20150087624, 2015.
Sharma RK, Yassin AEB. Nanostructure-based platforms-current prospective in ophthalmic drug delivery. Indian J Ophthalmol. 2014;62(7):768–72.
Pawar VK, Singh Y, Meher JG, Gupta S, Chourasia MK. Engineered nanocrystal technology: in-vivo fate, targeting and applications in drug delivery. J Control Release. 2014;183:51–66.
Müller RH, Shegokar R, Gohla S, Keck CM. Nanocrystals: production, cellular drug delivery, current and future products. Intracellular Delivery. Springer; 2011. pp. 411–32.
Xu Q, Kambhampati SP, Kannan RM. Nanotechnology approaches for ocular drug delivery. Middle East Afr J Ophthalmol. 2013;20(1):26–37.
Ravichandran R. Nanoparticles in drug delivery: potential green nanobiomedicine applications. Int J Green Nanotechnol Biomed. 2009;1(2):B108–30.
Zimmer A, Kreuter J. Microspheres and nanoparticles used in ocular delivery systems. Adv Drug Deliv Rev. 1995;16(1):61–73.
Wang Y, Zheng Y, Zhang L, Wang Q, Zhang D. Stability of nanosuspensions in drug delivery. J Control Release. 2013;172(3):1126–41.
Verma S, Kumar S, Gokhale R, Burgess DJ. Physical stability of nanosuspensions: investigation of the role of stabilizers on Ostwald ripening. Int J Pharm. 2011;406(1):145–52.
Ghosh I, Bose S, Vippagunta R, Harmon F. Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int J Pharm. 2011;409(1):260–8.
Kesisoglou F, Panmai S, Wu Y. Nanosizing—oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59(7):631–44.
Müller R, Keck C. Twenty years of drug nanocrystals: where are we, and where do we go? Eur J Pharm Biopharm. 2012;80(1):1–3.
Acknowledgments
Authors are also thankful to Prof. Anuradha K. Gajjar for her kind support in editing of manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Sharma, O.P., Patel, V. & Mehta, T. Nanocrystal for ocular drug delivery: hope or hype. Drug Deliv. and Transl. Res. 6, 399–413 (2016). https://doi.org/10.1007/s13346-016-0292-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-016-0292-0