Abstract
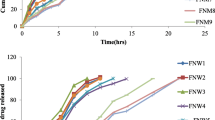
The present study is intended to enhance gastric retention of sustained release tablet of valacyclovir hydrochloride by combined approach of floating and swelling. The tablets are prepared by direct compression method. Polyethylene oxide (Polyox WSR 303) is selected as the swelling matrix agent. Sodium starch glycolate (SSG) is used as swelling enhancer, and sodium bicarbonate is used as an effervescent agent for floating. A 32 full factorial design is applied to systematically optimize the formulation. The concentration of Polyox WSR 303 (X 1) and concentration of SSG (X 2) are selected as independent variables. The percentage drug release at 12 h, floating lag time, and maximum percentage swelling are selected as dependent variables. Formulations are evaluated for hardness, friability, floating lag time, total floating time, percentage swelling, in vitro drug release, and in vivo floating study. The results indicated that X 1 and X 2 significantly affected the drug release properties, floating lag times, and maximum percentage swelling. Release rate decreases as the concentration of Polyox increased. Regression analysis and numerical optimization are performed to identify the best formulation. Formulation F5 prepared with Polyox WSR 303 (15 %) and SSG (10 %) is found to be the best formulation. F5 followed zero-order release mechanism. Swelling and floating gastroretentive tablets of valacyclovir HCl are successfully formulated with controlled delivery to stomach with an aim of increasing the mean residence time in the upper part of GIT where the drug has its absorption window.

The main objective is to formulate and evaluate swellable and floating tablets of a model drug valacyclovir HCl for sustained delivery to stomach with an aim of increasing the mean residence time in the stomach from where the drug slowly releases and reaches its absorption site at the upper part of the intestine. Thus, better absorption, improved bioavailability, reduction in side effects, local action, and also patient compliance of the product can enhance the efficiency of the medical treatment.





Similar content being viewed by others
References
Ummadi S, Shravani B, Rao R, et al. Overview on controlled release dosage form. Int J Pharm Sci. 2013;3(4):258–69.
Joshi G, Hemant K, Singh M, et al. Development of pH sensitive hydrogel for intestinal delivery of methyl prednisolone using novel chitosan derivative. Int J Pharm Pharma Sci. 2011;3(1):200–3.
Spandana A, Senthil S, Parthiban S. Formulation and evaluation of bilayer floating tablet containing verapamil hydrochloride. Int J Pharm Dev Tech. 2013;3(1):23–7.
Chikhalikar S, Wakade R. Floating drug delivery system—an approach to oral controlled drug delivery. Int J PharmTech Res. 2012;4(4):1812–26.
Nila M, Sudhir M, Cinu T, et al. Floating microspheres of carvedilol as gastro retentive drug delivery system: 32 full factorial design and in vitro evaluation. Drug Delivery. 2013. doi:10.3109/10717544.2013.834414.
Talukder R. Fassihi R, “Gastroretentive delivery systems: a mini review.”. Drug Dev Ind Pharm. 2004;30(10):1019–28.
Narang N. An updated review on Floating Drug Delivery System (FDDS). Int J Appl Pharm. 2011;3(1):1–7.
Bhimavarapu R, Nissankararao S, Nagavani S, et al. Design, development and in vitro evaluation of gastro retentive alginate floating beads for ranitidine hydrochloride. J Chem Pharma Res. 2013;5(4):377–81.
Varshosaz J, Tavakoli N, Roozbahani F. Formulation and in vitro characterization of ciprofloxacin floating and bioadhesive extended-release tablets. Drug Delivery. 2006;13:277–85.
Shah S, Pandya S. A novel approach in gastro retentive drug delivery system: floating drug delivery system. Int J Pharm Sci Res. 2010;1(6):7–18.
Patel J, Chavda J. Formulation and evaluation of glipizide floating-bioadhesive tablets. Braz Arch Biol Technol. 2010;53(5):1073–85.
Chaturvedi S, Kumari P, Singh S, et al. Approaches to increase the gastric residence time: floating drug delivery systems—a review. Asian J Pharm Clin Res. 2013;6(3):1–9.
Patel D, Patel M, Pandya N, et al. Gastroretentive drug delivery system of carbamazepine: formulation optimization using simplex lattice design: a technical note. AAPS PharmSciTech. 2007;8(1):E82–6.
Singh B, Kim K. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Release. 2000;63:235–59.
Nayak A, Maji R, Das B. Gastroretentive delivery systems: a review. Asian J Pharm Clin Res. 2010;3(1):2–10.
Pasternak B, Hviid A. Use of acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and the risk of birth defects. J Am Med Assoc. 2010;304(8):859–66.
Soul J, Seaber E. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob Agents Chemother. 1995;39:2759–64.
Kagan L, Hoffman A. Selection of drug candidates for gastroretentive dosage forms: pharmacokinetics following continuous intragastric mode. Eur J Pharm Biopharm. 2008;69:238–46.
Granero G, Amidon G. Stability of valacyclovir: implications for its oral bioavailability”. Int J Pharm. 2006;317:14–8.
Valacyclovir hydrochloride drug profile, Drug Bank, February 2012, http://drugbank.ca/drugs/DB00577.
Streubel A, Siepmann J, Bodmeier R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr Opin Pharmacol. 2006;6:501–8.
Lulu M, Deng L, Chen J. Applications of polyethylene oxide in controlled release tablet systems: a review. Drug Dev Ind Pharma. 2013. doi:10.3109/03639045.2013.831438.
Lachman L, Lieberman H, Kanig J. The theory and practice of industrial pharmacy; 3rd Edn; Varghese Publishing House. Bombay. 1991;293–294.
Government of India, Ministry of Health and Family Welfare. IP, Gaziabad; Indian Pharmacopoeia Commission, 2007, 2, 701–702.
Kumar P, Rathnam G, Prakash C, et al. Formulation and characterization of bilayer floating tablets of ranitidine. Rasayan J Chem. 2010;3(2):368–74.
Swarbrick J, Banakar V. Pharmaceutical dissolution testing, drug and the pharmaceutical sciences. New York: Marcel Dekker; 1992.
Londhe S, Gattani S, Surana S. Development of floating drug delivery system with biphasic release for verapamil hydrochloride: in vitro and in vivo evaluation. J Pharm Sci Tech. 2010;2(11):361–7.
Acknowledgments
The authors are thankful to LJK Trust, Ahmedabad, for providing the needed lab facilities. Authors are also thankful to Colorcon Asia Pvt. Ltd, Goa, India, for providing polyethylene oxide for the research work.
Conflict of interest
For the submission of this manuscript, we would like to undertake that Pratik Upadhyay, Kunal Nayak, Kaushika Patel, Jaymin Patel, Dr. Shreeraj Shah, and Prof. Jayant Deshpande declare that they have no conflict of interest. The research has been reviewed and approved by an appropriate human or animal research or ethics review committee ref: LJIP/IAEC/12-13/09. All authors agree that the contents of the manuscript are confidential and will not be copyrighted, submitted, or published elsewhere (including the Internet), in any language, while acceptance by the journal is under consideration.
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript has been submitted for publication in Drug Delivery and Translational Research. The corresponding author of this manuscript is Pratik H. Upadhyay and other contributors of the exertion are Kunal P. Nayak, Kaushika M. Patel, Jaymin M. Patel, Dr. Shreeraj H. Shah, and Prof. Jayant M. Deshpande with their responsibility in the research.
Rights and permissions
About this article
Cite this article
Upadhyay, P., Nayak, K., Patel, K. et al. Formulation development, optimization, and evaluation of sustained release tablet of valacyclovir hydrochloride by combined approach of floating and swelling for better gastric retention. Drug Deliv. and Transl. Res. 4, 452–464 (2014). https://doi.org/10.1007/s13346-014-0207-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-014-0207-x