Abstract
Introduction
To investigate changes in insulin requirements over time in patients who underwent hepatectomy and pancreatectomy with perioperative glycemic control by an artificial pancreas (STG-55).
Materials and methods
We included 56 patients (22 hepatectomies and 34 pancreatectomies) who were treated with an artificial pancreas in the perioperative period and investigated the differences in insulin requirements by organ and surgical procedure.
Results
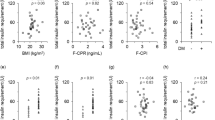
The mean intraoperative blood glucose level and total insulin doses were higher in the hepatectomy group than in the pancreatectomy group. The dose of insulin infusion increased in hepatectomy, especially early in surgery, compared to pancreatectomy. In the hepatectomy group, there was a significant correlation between the total intraoperative insulin dose and Pringle time, and in all cases, there was a correlation with surgical time, bleeding volume, preoperative CPR, preoperative TDD, and weight.
Conclusions
Perioperative insulin requirements may be mainly dependent on the surgical procedure, invasiveness, and organ. Preoperative prediction of insulin requirements for each surgical procedure contributes to good perioperative glycemic control and improvement of postoperative outcomes.



Similar content being viewed by others
Change history
28 April 2023
A Correction to this paper has been published: https://doi.org/10.1007/s13340-023-00632-2
References
Kazuhiro H. Latest clinically useful blood glucose control manual. Saitama: Medical Book Publishing; 2012. p. 89–95.
Quagliaro L, Piconi L, Assloni R. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cell: the role of protein kinase C and NAD (P) H-oxidase activation. Diabetes. 2003;52:2795–804.
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–67.
Ata A, Lee J, Bestle S, Desemone J, Stain S. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg. 2010;145:858–64.
The NICE-SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367:1108–18.
Lee A, Faddoul B, Sowan A, Johnson K, Silver K, Vaidya V. Computerisation of a paper-based intravenous insulin protocol reduces errors in a prospective crossover simulated tight glycemic control study. Intensive Crit Care Nursing. 2010;26:161–8.
Yatabe T, Yamazaki R, Kitagawa H, Takehiro O, Koichi Y, Kazuhiro H, Masataka Y. The evaluation of the ability of closed-loop glycemic control device to maintain the blood glucose concentration in intensive care unit patients. Crit Care Med. 2011;39:575–8.
Watanabe K, Maeda H, Shojima S, Takeda M, Nakatou T, Fushimi T, Kojima T, Nikuma T, Akazai Y, Mimura T. Perioperative blood glucose for the third hepatectomy. A case of type 1 diabetes with a favorable course using an artificial pancreas (STG-55) for management. Diabetes. 2018;61(9):593–9.
Okabayashi T, Nishimori I, Maeda H, Yamashita K, Yatabe T, Hanazaki K. Effect of intensive insulin therapy using a closed-loop glycemic control system in hepatic resection patients. Diabetes Care. 2009;32:1425–7.
Hanazaki K, Kitagawa H, Yatabe T, Munekage M, Dabanaka K, Takezaki Y, Tsukamoto Y, Asano T, Kinoshita Y, Namikawa T. Perioperative intensive insulin therapy using an artificial endocrine pancreas with closed-loop glycemic control system: the effects of no hypoglycemia. Am J Surg. 2014;207(6):935–41.
Hanazaki K, Munekage M, Kitagawa H, Yatabe T, Munekage E, Shiga M, Maeda H, Namikawa T. Current topics in glycemic control by wearable artificial pancreas or bedside artificial pancreas with closed-loop system. J Artif Organs. 2016;19:209–18.
Hanazaki K, Maeda H, Okabayashi T. Relationship between perioperative glycemic control and postoperative infections. World J Gastroenterol. 2009;15:4122–5.
Okabayashi T, Maeda H, Zhao-Li SZI, Montgomery RA, Nishimori I, Hanazaki K. Perioperative insulin therapy using a closed-loop artificial endocrine pancreas after hepatic resection. World J Gastroenterol. 2009;15(33):4116–21.
Sawa A, Kimura K, Morikane K, Harihara Y. Examination of SSI risk factors in the field of gastrointestinal surgery in Japan based on JHAIS data. J Jpn Dept Surg Infect Dis. 2013;10(1):43–52.
Murakami M, Junzo Shimizu J, Koga M, Kawabata R, Noura S, Hasegawa J. Examination of postoperative hyperglycemia and surgical site infection in hepatobiliary and pancreatic surgery. J Jpn Soc Surg Infect Dis. 2015;12(6):657–61.
Maeda H, Okabayashi T, Yatabe T, Yamashita K, Hanazaki K. Perioperative intensive insulin therapy using artificial endocrine pancreas in patients undergoing pancreatectomy. World J Gastroenterol. 2009;15(33):4111–5.
Obayashi T, Nishimori I, Yamashita K, Sugimoto T, Maeda H, Yatabe T, Kohsaki T, Kobayashi M, Hanazaki K. Continuous postoperative blood glucose monitoring and control by artificial pancreas in patients having pancreatic resection. Arch Surg. 2009;144(10):933–7.
Pringle JH. Note on the arrest of hepatic hemorrhage due to trauma. Ann Surg. 1908;48:541.
Figueras J, Llado L, Ruiz D, Ramos E, Busquets J, Rafecas A, Torras J, Fabregat J. Complete versus selective portal triad clamping for minor liver resections: a prospective randomized trial. Ann Surg. 2005;241:582–90.
Ishizaki Y, Yoshimoto J, Miwa K, Sugo H, Kawasaki S. Safety of prolonged intermittent Pringle maneuver during hepatic resection. Arch Surg. 2006;141:649–53.
Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli A, Marty J, Farges O. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369–75.
Maeda H, Okabayashi T, Nishimori I, Yamashita K, Sugimoto T, Hanazaki K. Hyperglycemia during hepatic resection: continuous monitoring of blood glucose concentration. Am J Surg. 2010;199:8–13.
Funding
Sanofi K.K., Eli Lilly Japan, Abbott Japan, Novo Nordisk Pharma, Dainippon Sumitomo Pharma.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Atsuhito T. received lecture fees from Sanofi, Eli Lilly, and Abbott. Author Tatsuaki N. received lecture fees from Eli Lilly Japan, Novo Nordisk Pharma, and Sumitomo Dainippon Pharma. Other authors declare that they have no conflict of interest associated with this research.
Ethical approval and consent to participate
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. This study was approved by the ethics committee of Okayama Saiseikai General Hospital (approval no. 190303; approval date February 25th, 2019). We did not receive informed consent, but we provided the participants with the opportunity to refuse by publishing the Opt Out document.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Teshigawara, S., Tone, A., Katayama, A. et al. Time course change of the insulin requirements during the perioperative period in hepatectomy and pancreatectomy by using an artificial pancreas STG-55. Diabetol Int 14, 262–270 (2023). https://doi.org/10.1007/s13340-023-00623-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-023-00623-3