Abstract
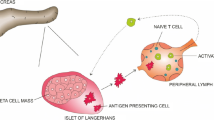
Diabetes is a major health problem worldwide. It is a chronic metabolic disorder that produces overt hyperglycemic condition that occurs either when the pancreas does not produce enough insulin due to excessive destruction of pancreatic β-cells (type 1 diabetes) or due to development of insulin resistance (type 2 diabetes). An autoimmune condition known as type 1 diabetes (T1D) results in the targeted immune death of β-cells that produce insulin. The only available treatment for T1D at the moment is the lifelong use of insulin. Multiple islet autoantibody positivity is used to diagnose T1D. There are four standard autoantibodies observed whose presence shows the development of T1D: antibodies against insulin, glutamic acid decarboxylase (GAD65), zinc T8 transporter (ZnT8), and tyrosine phosphatase-like protein (ICA512). In type 2 diabetes (T2D), an inflammatory response precipitates as a consequence of the immune response to high blood glucose level along with the presence of inflammation mediators produced by macrophages and adipocytes in fat tissue. The slow and chronic inflammatory condition of adipose tissue produces insulin resistance leading to increased stress on pancreatic β-cells to produce more insulin to compensate for the insulin resistance. Thus, this stress condition exacerbates the apoptosis of β-cells leading to insufficient production of insulin, resulting in hyperglycemia which signifies late stage T2D. Therefore, the therapeutic utilization of immunosuppressive agents may be a better alternative over the use of insulin and oral hypoglycemic agents for the treatment of T1D and T2D, respectively. This review enlightens the immune intervention for the prevention and amelioration of T1D and T2D in humans with main focus on the antigen-specific immune suppressive therapy.


Similar content being viewed by others
References
Inzucchi SE. Diagnosis of diabetes. N Engl J Med. 2013;368:193.
Alam U, Asghar O, Azmi S, Malik RA. General aspects of diabetes mellitus. Handb Clin Neurol. 2014;126:211–22.
American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes – 2018. Diabetes Care. 2018;41:S13-27.
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14.
Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–40.
Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801.
Phillips JM, Parish NM, Raine T, et al. Type 1 diabetes development requires both CD4+ and CD8+ T cells and can be reversed by non-depleting antibodies targeting both T cell populations. Rev Diabet Stud. 2009;6:97–103.
Richardson SJ, Morgan NG, Foulis AK. Pancreatic pathology in type 1 diabetes mellitus. Endocr Pathol. 2014;25:80–92.
Willcox A, Richardson SJ, Bone AJ, et al. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155:173–81.
Feuerer M, Shen Y, Littman DR, et al. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 2009;31:654–64.
Pirot P, Eizirik DL, Cardozo AK. Interferon-gamma potentiates endoplasmic reticulum stress-induced death by reducing pancreatic beta cell defence mechanisms. Diabetologia. 2006;49:1229–36.
Saad MF, Knowler WC, Pettitt DJ, et al. Sequential changes in serum insulin concentration during development of noninsulin-dependent diabetes. Lancet. 1989;1:1356–9.
Tabák AG, Jokela M, Akbaraly TN, et al. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373:2215–21.
Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and betacell function in human subjects. Evid Hyperb Funct Diabetes. 1993;42:1663–7.
Uysal KT, Wiesbrock SM, Marino MW, et al. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–4.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7.
Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46.
Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7.
Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91.
Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56.
Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am. 2008;37:753–68.
Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes. 2009;33:54–66.
Gao Z, He Q, Peng B, et al. Regulation of nuclear translocation of HDAC3 by IkappaBalpha is required for tumor necrosis factor inhibition of peroxisome proliferator-activated receptor gamma function. J Biol Chem. 2006;281:4540–7.
Gao Z, Hwang D, Bataille F, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115–21.
Aguirre V, Uchida T, Yenush L, et al. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem. 2000;275:9047–54.
Zhang J, Gao Z, Yin J, et al. S6K directly phosphorylates IRS-1 on Ser-270 to promote insulin resistance in response to TNF-(alpha) signaling through IKK2. J Biol Chem. 2008;283:35375–82.
Rui L, Aguirre V, Kim JK, et al. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 2001;107:181–9.
Sacks H, Symonds ME. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes. 2013;62:1783–90.
Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015;125:478–86.
Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–80.
Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494505.
Antonopoulos AS, Margaritis M, Coutinho P, et al. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes. 2015;64:2207–19.
Antoniades C, Antonopoulos AS, Tousoulis D, et al. Adiponectin: from obesity to cardiovascular disease. Obes Rev. 2009;10:269–79.
Burcelin R, Garidou L, Pomie C. Immuno-microbiota cross and talk: the new paradigm of metabolic diseases. Semin Immunol. 2012;24:67–74.
Cani PD, Osto M, Geurts L, et al. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3:279–88.
Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72.
Hersoug LG, Møller S, Loft S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: implications for inflammation and obesity. Obes Rev. 2016;17:297–312.
Remely M, Aumueller E, Merold C, et al. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene. 2014;537:85–92.
Alvarez-Curto E, Milligan G. Metabolism meets immunity: the role of free fatty acid receptors in the immune system. Biochem Pharmacol. 2016;114:3–13.
Scheithauer TP, Dallinga-Thie GM, de Vos WM, et al. Causality of small and large intestinal microbiota in weight regulation and insulin resistance. Mol Metab. 2016;5:759–70.
Nastasi C, Candela M, Bonefeld CM, et al. The effect of short chain fatty acids on human monocyte-derived dendritic cells. Sci Rep. 2015;5:16148.
Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–5.
Cavelti-Weder C, Babians-Brunner A, Keller C, et al. Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care. 2012;35:1654–62.
Sauter NS, Schulthess FT, Galasso R, et al. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology. 2008;149:2208–18.
Marchetti P, Suleiman M, De Luca C, et al. A direct look at the dysfunction and pathology of the β cells in human type 2 diabetes. Semin Cell Dev Biol. 2020;103:83–93.
Galluzzi L, Bravo-San Pedro JM, Vitale I, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22:58–73.
Ardestani A, Li S, Annamalai K, et al. Neratinib protects pancreatic beta cells in diabetes. Nat Commun. 2019;10:1–17.
Ardestani A, Maedler K. MST1: a promising therapeutic target to restore functional beta cell mass in diabetes. Diabetologia. 2016;59:1843–9.
Yuan T, Annamalai K, Naik S, et al. The Hippo kinase LATS2 impairs pancreatic β-cell survival in diabetes through the mTORC1-autophagy axis. Nat Commun. 2021;12:1–18.
Rausch V, Hansen CG. The Hippo pathway, YAP/TAZ, and the plasma membrane. Trends Cell Biol. 2020;30:32–48.
Chang YC, Wu JW, Wang CW, et al. Hippo signaling-mediated mechanotransduction in cell movement and cancer metastasis. Front Mol Biosci. 2020;6:1–7.
Wu S, Huang J, Dong J, et al. Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–56.
Yu F, Jiang R, Han W, et al. Gut microbiota transplantation from db/db mice induces diabetes-like phenotypes and alterations in Hippo signaling in pseudo germ-free mice. Aging. 2020;12:24156–67.
Ardestani A, Maedler K. The Hippo signaling pathway in pancreatic β-cells: functions and regulations. Endocr Rev. 2018;39:21–35.
Zhao B, Li L, Lei Q, et al. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862–74.
Fallahi E, O’Driscoll NA, Matallanas D. The MST/Hippo pathway and cell death: a non-canonical affair. Genes. 2016;7:28.
Matallanas D, Romano D, Hamilton G, et al. A Hippo in the ointment: MST signalling beyond the fly. Cell Cycle. 2008;7:879–84.
Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in drosophila and mammals. Cell. 2009;130:1120–33.
Maugeri-Saccàa M, De Maria R. The Hippo pathway in normal development and cancer. Pharmacol Ther. 2018;186:60–72.
Misra JR, Irvine KD. The hippo signaling network and its biological functions. Annu Rev Genet. 2018;52:65–87.
Al-Nahdi AMT, John A, Haider R. Cytoprotective effects of N-acetylcysteine on streptozotocin-induced oxidative stress and apoptosis in RIN-5F pancreatic. Cell Physiol Biochem. 2018;51:201–16.
Ardestani A, Paroni F, Azizi Z, et al. MST1 is a novel regulator of apoptosis in pancreatic beta-cells. Nat Med. 2014;20:385–97.
Yuan T, Maedler K, Ardestani A. Pancreatic β-cell rescue in diabetes by targeting Merlin. Expert Rev Endocrinol Metab. 2017;12:97–9.
Liu J, Li J, Chen H, et al. Metformin suppresses proliferation and invasion of drug-resistant breast cancer cells by activation of the Hippo pathway. J Cell Mol Med. 2020;24:5786–96.
Shu Z, Gao Y, Zhang G, et al. A functional interaction between Hippo-YAP signalling and SREBPs mediates hepatic steatosis in diabetic mice. J Cell Mol Med. 2019;23:3616–28.
Weiner HL. Oral tolerance, an active immunologic process mediated by multiple mechanisms. J Clin Invest. 2000;106:935–7.
Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. The TrialNet Natural History Study of the development of type 1 diabetes: objectives, design, and initial results. Pediatr Diabetes. 2009;10:97–104.
Skyler JS, Brown D, Chase HP, et al. Effects of insulin in relatives of patients with type 1 diabetes mellitus. New Engl J Med. 2002;346:1685–91.
Nanto-Salonen K, Kupila A, Simell S, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 2008;372:1746–55.
Nakayama M, Abiru N, Moriyama H, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–3.
Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med. 2009;360:1646–54.
Wicklow BA, Polychronakos C. Insulin auto-immunity: implications for the prevention of type 1 diabetes mellitus. Expert Rev Clin Immunol. 2009;5:55–62.
Barratt BJ, Payne F, Lowe CE, et al. Remapping the insulin gene/IDDM2 locus in type 1 diabetes. Diabetes. 2004;53:1884–9.
Burrack AL, Martinov T, Fife BT. T cell-mediated beta cell destruction: autoimmunity and alloimmunity in the context of type 1 diabetes. Front Endocrinol (Lausanne). 2017;8:343.
Cabello-Olmo M, Araña M, Radichev I, et al. New insights into immunotherapy strategies for treating autoimmune diabetes. Int J Mol Sci. 2019;20:4789.
Rathod S. Novel insights into the immunotherapy-based treatment strategy for autoimmune type 1 diabetes. Diabetology. 2022;3:79–96.
Taplin CE, Barker JM. Autoantibodies in type 1 diabetes. Autoimmunity. 2008;41:11–8.
Roep BO, Peakman M. Antigen targets of type 1 diabetes autoimmunity. Cold Spring Harb Perspect Med. 2012;2: a007781.
Espinosa-Carrasco G, Le Saout C, Fontanaud P, et al. CD4+ T helper cells play a key role in maintaining diabetogenic CD8+ T cell function in the pancreas. Front Immunol. 2018;8:2001.
Anderson AM, Landry LG, Alkanani AA, et al. Human islet T cells are highly reactive to preproinsulin in type 1 diabetes. Proc Natl Acad Sci U S A. 2021;118: e2107208118.
Usmani-Brown S, Perdigoto AL, Lavoie N, et al. β cell responses to inflammation. Mol Metab. 2019;27S:S104–13.
Chatenoud L, Warncke K, Ziegler AG. Clinical immunologic interventions for the treatment of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2: a007716.
Paroni F, Domsgen E, Maedler K. CXCL10- a path to β-cell death. Islets. 2009;1:256–9.
Turner MD, Nedjai B, Hurst T, et al. Cytokines and chemokines : at the crossroads of cell signalling and inflammatory disease. Biochimica et Biophysica Acta (BBA)–Mol Cell Res. 2014;1843:2563–82.
Aschner P, Kipnes MS, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–7.
Yamagishi S, Fukami K, Matsui T. Crosstalk between advanced glycation end products (AGEs)-receptor RAGE axis and dipeptidyl peptidase-4-incretin system in diabetic vascular complications. Cardiovasc Diabetol. 2015;14:1–12.
Yaron A, Naider F. Proline-dependent structural and biological properties of peptides and proteins. Crit Rev Biochem Mol Biol. 1993;28:31–81.
Ohnuma K, Yamochi T, et al. CD26 mediates dissociation of Tollip and IRAK-1 from caveolin-1 and induces upregulation of CD86 on antigen-presenting cells. Mol Cell Biol. 2005;25:7743–57.
Hiromura M, Nohtomi K, et al. Caveolin-1, a binding protein of CD26, is essential for the anti-inflammatory effects of dipeptidyl peptidase-4 inhibitors on human and mouse macrophages. Biochem biophys res commun. 2018;495:223–9.
Guo Q, Zhang S, et al. Alogliptin inhibits IL-1β-induced inflammatory response in fibroblast-like synoviocytes. Int Immunopharmacol. 2020;83: 106372.
Kume S, Takeya M, et al. Immunohistochemical and ultrastructural detection of advanced glycation end products in atherosclerotic lesions of human aorta with a novel specific monoclonal antibody. Am J Pathol. 1995;147:654–67.
Kajikawa M, Nakashima A, et al. Ratio of serum levels of AGEs to soluble form of RAGE is a predictor of endothelial function. Diabetes Care. 2015;38:119–25.
Allahverdian S, Pannu PS, et al. Contribution of monocyte-derived macrophages and smooth muscle cells to arterial foam cell formation. Cardiovasc Res. 2012;95:165–72.
Terasaki M, Yashima H, et al. A dipeptidyl peptidase-4 inhibitor inhibits foam cell formation of macrophages in type 1 diabetes via suppression of CD36 and ACAT-1 expression. Int J Mol Sci. 2020;21:4811.
Liadis N, Murakami K, et al. Caspase-3-dependent β-cell apoptosis in the initiation of autoimmune diabetes mellitus. Mol Cell Biol. 2005;25:3620–9.
Samaha MM, Said E, et al. A comparative study of the role of crocin and sitagliptin in attenuation of STZ-induced diabetes mellitus and the associated inflammatory and apoptotic changes in pancreatic β-islets. Environ toxicol pharmacol. 2019;72: 103238.
Mahabadi-Ashtiyani E, Sheikh V, et al. The increased T helper cells proliferation and inflammatory responses in patients with type 2 diabetes mellitus is suppressed by sitagliptin and vitamin D3 in vitro. Inflamm Res. 2019;68:857–66.
Borzouei S, Sheikh V, et al. Anti-Inflammatory effect of combined sitagliptin and vitamin D3 on cytokines profile in patients with type 2 diabetes mellitus. J Interferon Cytokine Res. 2019;39:293–301.
Li Y, Zhang Z, Yang L, et al. The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike. iScience. 2020;23:101160.
Vankadari N, Wilce JA. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9:601–4.
Pinheiro MM, et al. Cytokine storm modulation in COVID-19: a proposed role for vitamin D and DPP-4 inhibitor combination therapy (VIDPP-4i). Immunotherapy. 2021;13:753–65.
Pal R, Banerjee M, et al. Dipeptidyl peptidase-4 inhibitor use and mortality in COVID-19 patients with diabetes mellitus: an updated systematic review and meta-analysis. Ther Adv Endocrinol Metab. 2021;12:2042018821996482.
Yuan M, et al. Reversal of obesity-and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science. 2001;293:1673–7.
Shoelson SE, Lee J, et al. Inflammation and the IKKβ/IκB/NF-κB axis in obesity and diet-induced insulin resistance. Int J Obes Relat Metab Disord. 2003;27:S49-52.
Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333.
Akira S, Uematsu S, et al. Pathogen recognition and innate immunity. Cell. 2006;124:783–801.
Lee JY, Sohn KH, et al. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–9.
Ramasamy R, Yan SF, et al. The RAGE axis and endothelial dysfunction: maladaptive roles in the diabetic vasculature and beyond. Trends Cardiovasc Med. 2005;15:237–43.
Pascual G, Fong AL, et al. A SUMOylation-dependent pathway mediates trans repression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–63.
Lalenti A, Grassia G, et al. Mechanism of the anti-inflammatory effect of thiazolidinediones: relationship with the glucocorticoid pathway. Mol Pharmacol. 2005;67:1620–8.
Evans JM, Ogston SA, et al. Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia. 2006;49:930–6.
UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–65.
Clark K, Peggie M, et al. Novel cross-talk within the IKK family controls innate immunity. Biochem J. 2011;434:93–104.
Cameron AR, Morrison VL, et al. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ Res. 2016;119:652–65.
Castrillo A, Tontonoz P. Nuclear receptors in macrophage biology: at the crossroads of lipid metabolism and inflammation. Annu Rev Cell Dev Biol. 2004;20:455–80.
Hundal RS, Petersen KF, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–6.
Kopp E, Ghosh S. Inhibition of NF kappa B by sodium salicylate and aspirin. Science. 1994;265:956–9.
Yin MJ, Yamamoto Y, et al. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature. 1998;396:77–80.
Thompson PL, Nidorf SM, et al. Anti-inflammatory therapy with canakinumab for atherosclerotic disease: lessons from the CANTOS trial. J Thorac Dis. 2018;10:695–8.
Everett BM, Donath MY, et al. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J Am Coll Cardiol. 2018;71:2392–401.
Kataria Y, Ellervik C, et al. Treatment of type 2 diabetes by targeting interleukin-1: a meta-analysis of 2921 patients. Semin Immunopathol. 2019;41:413–25.
Dominguez H, Storgaard H, et al. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res. 2005;42:517–25.
Gonzalez-Gay MA, De Matias JM, et al. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83–6.
Ban E, Jeong S, et al. Accelerated wound healing in diabetic mice by miRNA-497 and its anti-inflammatory activity. Biomed Pharmacother. 2020;121: 109613.
Rissanen A, Howard CP, Botha J, et al. Effect of anti-IL-1beta antibody (canakinumab) on insulin secretion rates in impaired glucose tolerance or type 2 diabetes: results of a randomized, placebo-controlled trial. Diabetes Obes Metab. 2012;14:1088–96.
van Asseldonk EJ, Stienstra R, Koenen TB, et al. Treatment with Anakinra improves disposition index but not insulin sensitivity in nondiabetic subjects with the metabolic syndrome: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2011;96:2119–26.
Sloan-Lancaster J, Abu-Raddad E, Polzer J, et al. Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1beta antibody, in patients with type 2 diabetes. Diabetes Care. 2013;36:2239–46.
Fleischman A, Shoelson SE, Bernier R, et al. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–94.
Kiortsis DN, Mavridis AK, Vasakos S, et al. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64:765–6.
Ramos-Zavala MG, González-Ortiz M, Martínez-Abundis E, et al. Effect of diacerein on insulin secretion and metabolic control in drug-naive patients with type 2 diabetes: a randomized clinical trial. Diabetes Care. 2011;34:1591–4.
Stanley TL, Zanni MV, Johnsen S, et al. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E146–50.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest. This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Rai, U., Senapati, D. & Arora, M.K. Insights on the role of anti-inflammatory and immunosuppressive agents in the amelioration of diabetes. Diabetol Int 14, 134–144 (2023). https://doi.org/10.1007/s13340-022-00607-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-022-00607-9