Abstract
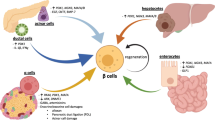
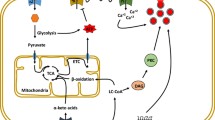
The structural, functional, and pathological differences between human islets and rodent islets, such as mouse or rat islets, have been clarified, and research using human islets is becoming more important for elucidating the pathophysiology of diabetes and developing therapeutic strategies for diabetes. Increasing the functional human β-cell mass is a feasible method for the treatment of both type 1 and type 2 diabetes. The glucokinase-mediated glucose signaling pathway is known to promote β-cell proliferation not only in rodent models but also in humans. However, little is known about the signaling components of glucose- or glucokinase-mediated signaling pathways. Studies have gradually revealed the involvement of ER stress-related molecules, cell cycle regulators, inflammatory proteins, extracellular matrix proteins, and neurotransmitters in the glucokinase-mediated signaling pathway in β-cells. Unraveling the mechanisms of those molecules in the regulation of human β-cell mass will provide new insights into how the functional β-cell mass can be increased. The human islet distribution program is essential for human islet research. However, human islets for research are only available by import from Europe and America into Japan or Asia. Since Japanese or Asian diabetes patients possibly show different features compared with European or American diabetes patients, studies using human islets from Japanese or Asian populations have become important for the elucidation of pathophysiology in these specific population groups. This review outlines new pathways important for the regulation of human pancreatic β-cell mass and expectations regarding the establishment of a human islet distribution program in Japan.




Similar content being viewed by others
References
Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005;48(11):2221–8.
Keenan HA, Sun JK, Levine J, Doria A, Aiello LP, Eisenbarth G, et al. Residual insulin production and pancreatic ss-cell turnover after 50 years of diabetes: Joslin medalist study. Diabetes. 2010;59(11):2846–53.
Yu MG, Keenan HA, Shah HS, Frodsham SG, Pober D, He Z, et al. Residual beta cell function and monogenic variants in long-duration type 1 diabetes patients. J Clin Invest. 2019;129(8):3252–63.
Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–10.
Hart NJ, Powers AC. Use of human islets to understand islet biology and diabetes: progress, challenges and suggestions. Diabetologia. 2019;62(2):212–22.
Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103(7):2334–9.
van der Meulen T, Donaldson CJ, Caceres E, Hunter AE, Cowing-Zitron C, Pound LD, et al. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat Med. 2015;21(7):769–76.
Kaddis JS, Olack BJ, Sowinski J, Cravens J, Contreras JL, Niland JC. Human pancreatic islets and diabetes research. JAMA. 2009;301(15):1580–7.
Kulkarni RN, Stewart AF. Summary of the Keystone islet workshop (April 2014): the increasing demand for human islet availability in diabetes research. Diabetes. 2014;63(12):3979–81.
Levitt HE, Cyphert TJ, Pascoe JL, Hollern DA, Abraham N, Lundell RJ, et al. Glucose stimulates human beta cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia. 2011;54(3):572–82.
Shirakawa J, Terauchi Y. Newer perspective on the coupling between glucose-mediated signaling and beta-cell functionality. Endocr J. 2020;67(1):1–8.
Kassem S, Bhandari S, Rodriguez-Bada P, Motaghedi R, Heyman M, Garcia-Gimeno MA, et al. Large islets, beta-cell proliferation, and a glucokinase mutation. N Engl J Med. 2010;362(14):1348–50.
Shirakawa J, Tanami R, Togashi Y, Tajima K, Orime K, Kubota N, et al. Effects of liraglutide on beta-cell-specific glucokinase-deficient neonatal mice. Endocrinology. 2012;153(7):3066–75.
Kawamori D, Shirakawa J, Liew CW, Hu J, Morioka T, Duttaroy A, et al. GLP-1 signalling compensates for impaired insulin signalling in regulating beta cell proliferation in betaIRKO mice. Diabetologia. 2017;60(8):1442–53.
Shirakawa J, Fujii H, Ohnuma K, Sato K, Ito Y, Kaji M, et al. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes. 2011;60(4):1246–57.
Shirakawa J, Amo K, Ohminami H, Orime K, Togashi Y, Ito Y, et al. Protective effects of dipeptidyl peptidase-4 (DPP-4) inhibitor against increased beta cell apoptosis induced by dietary sucrose and linoleic acid in mice with diabetes. J Biol Chem. 2011;286(29):25467–76.
Shirakawa J, Togashi Y, Sakamoto E, Kaji M, Tajima K, Orime K, et al. Glucokinase activation ameliorates ER stress-induced apoptosis in pancreatic beta-cells. Diabetes. 2013;62(10):3448–58.
Gunton JE, Kulkarni RN, Yim S, Okada T, Hawthorne WJ, Tseng YH, et al. Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;122(3):337–49.
Shirakawa J, Fernandez M, Takatani T, El Ouaamari A, Jungtrakoon P, Okawa ER, et al. Insulin signaling regulates the FoxM1/PLK1/CENP-A pathway to promote adaptive pancreatic beta cell proliferation. Cell Metab. 2017;25(4):868–82.
Shirakawa J, Okuyama T, Yoshida E, Shimizu M, Horigome Y, Tuno T, et al. Effects of the antitumor drug OSI-906, a dual inhibitor of IGF-1 receptor and insulin receptor, on the glycemic control, beta-cell functions, and beta-cell proliferation in male mice. Endocrinology. 2014;155(6):2102–11.
Shirakawa J, Tajima K, Okuyama T, Kyohara M, Togashi Y, De Jesus DF, et al. Luseogliflozin increases beta cell proliferation through humoral factors that activate an insulin receptor- and IGF-1 receptor-independent pathway. Diabetologia. 2020;63(3):577–87.
Inoue H, Shirakawa J, Togashi Y, Tajima K, Okuyama T, Kyohara M, et al. Signaling between pancreatic beta cells and macrophages via S100 calcium-binding protein A8 exacerbates beta-cell apoptosis and islet inflammation. J Biol Chem. 2018;293(16):5934–46.
Ji Y, Sun S, Shrestha N, Darragh LB, Shirakawa J, Xing Y, et al. Toll-like receptors TLR2 and TLR4 block the replication of pancreatic beta cells in diet-induced obesity. Nat Immunol. 2019;20(6):677–86.
Okuyama T, Shirakawa J, Yanagisawa H, Kyohara M, Yamazaki S, Tajima K, et al. Identification of the matricellular protein Fibulin-5 as a target molecule of glucokinase-mediated calcineurin/NFAT signaling in pancreatic islets. Sci Rep. 2017;7(1):2364.
Acknowledgements
A summary of this review was presented in the Lilly Award Lecture at the 64th Annual Meeting of the Japan Diabetes Society, Kanazawa, Japan. I would like to express sincere gratitude to Professor Yasuo Terauchi (Yokohama City University) and Professor Rohit N. Kulkarni (Joslin Diabetes Center) for their mentoring and to my colleagues and collaborators for their support. The author was supported by JSPS KAKENHI (20K08866) and AMED SICORP (192B9002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jun Shirakawa declares that he has no conflicts of interest associated with this research.
Human or animal rights
This article does not include data collected from any studies involving human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Shirakawa, J. Translational research on human pancreatic β-cell mass expansion for the treatment of diabetes. Diabetol Int 12, 349–355 (2021). https://doi.org/10.1007/s13340-021-00531-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-021-00531-4