Abstract
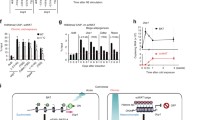
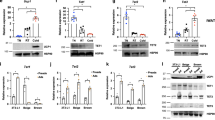
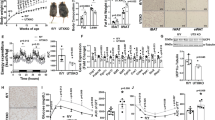
Adipocytes play a pivotal role in the regulation of energy metabolism. While white adipocyte stores energy, brown adipocyte dissipates energy by producing heat. In addition, another type of heat-producing adipocyte, beige adipocyte, emerges in white adipose tissue in response to chronic coldness. This phenotypic adaptation to the cold environment is considered to be attributed to the epigenetic modifications. Histone methylation is a chemically stable epigenetic modification and thus a proper mechanism for long-lasting cellular memory. Several histone methyl-modifying enzymes such as EHMT1, JMJD1A, JMJD3, and LSD1 are reported to be involved in the beige adipose cell fate determination. Among these, a histone demethylase JMJD1A senses cold environment by being phosphorylated at S265 in response to β-adrenergic receptor stimulation. Phosphorylated JMJD1A regulates both acute and cold thermogenesis. Under acute coldness, phosphorylated JMJD1A forms a complex with chromatin remodeler SWI/SNF and DNA-bound PPARγ, which recruits JMJD1A to the target genomic regions in brown adipocyte. This complex formation, in turn, induces the expression of target genes by bringing the enhancer and the promoter into close proximity. During chronic coldness, phosphorylated JMJD1A regulates beige adipogenesis through a two-step mechanism. In the first step, phosphorylated JMJD1A is recruited to the regulatory regions of target genes by forming a complex with PRDM16, PGC1α, and DNA-bound PPARγ. In the second step, JMJD1A demethylates histone H3K9me2 and induces stable expression of beige-selective genes. The phenotypic analyses of Jmjd1a-null mice and non-phosphorylated mutant S265A Jmjd1a knock-in mice indicate that JMJD1A is a potential therapeutic target for the treatment of obesity-related diseases including metabolic syndrome and type 2 diabetes.



Similar content being viewed by others
References
Shi Y, Lan F, Matson C, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;7:941–53.
Bannister AJ, Schneider R, Kouzarides T. Histone methylation: dynamic or static? Cell. 2002;7:801–6.
Bannister AJ, Kouzarides T. Reversing histone methylation. Nature. 2005;7054:1103–6.
Inagaki T, Sakai J, Kajimura S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat Rev Mol Cell Biol. 2016;8:480–95.
Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;7207:961–7.
Kajimura S, Seale P, Kubota K, et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;7259:1154–8.
Ohno H, Shinoda K, Ohyama K, et al. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature. 2013;7478:163–7.
Ogawa H, Ishiguro K, Gaubatz S, et al. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science. 2002;5570:1132–6.
Kaukonen R, Mai A, Georgiadou M, et al. Normal stroma suppresses cancer cell proliferation via mechanosensitive regulation of JMJD1a-mediated transcription. Nat Commun. 2016;7:12237.
Pan D, Huang L, Zhu LJ, et al. Jmjd3-mediated H3K27me3 dynamics orchestrate brown fat development and regulate white fat plasticity. Dev Cell. 2015;5:568–83.
Bernstein BE, Mikkelsen TS, Xie X, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;2:315–26.
Matsumura Y, Nakaki R, Inagaki T, et al. H3K4/H3K9me3 bivalent chromatin domains targeted by lineage-specific DNA methylation pauses adipocyte differentiation. Mol Cell. 2015;4:584–96.
Zeng X, Jedrychowski MP, Chen Y, et al. Lysine-specific demethylase 1 promotes brown adipose tissue thermogenesis via repressing glucocorticoid activation. Genes Dev. 2016;16:1822–36.
Lin JZ, Farmer SR. LSD1-a pivotal epigenetic regulator of brown and beige fat differentiation and homeostasis. Genes Dev. 2016;16:1793–5.
Duteil D, Tosic M, Willmann D, et al. Lsd1 prevents age-programed loss of beige adipocytes. Proc Natl Acad Sci USA. 2017;20:5265–70.
Sambeat A, Gulyaeva O, Dempersmier J, et al. LSD1 interacts with Zfp516 to promote UCP1 transcription and brown fat program. Cell Rep. 2016;11:2536–49.
Yamane K, Toumazou C, Tsukada Y, et al. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell. 2006;3:483–95.
Abe Y, Rozqie R, Matsumura Y, et al. JMJD1A is a signal-sensing scaffold that regulates acute chromatin dynamics via SWI/SNF association for thermogenesis. Nat Commun. 2015;6:7052.
Abe Y, Fujiwara Y, Takahashi H, et al. Histone demethylase JMJD1A coordinates acute and chronic adaptation to cold stress via thermogenic phospho-switch. Nat Commun. 2018;9:1566.
Inagaki T, Tachibana M, Magoori K, et al. Obesity and metabolic syndrome in histone demethylase JHDM2a-deficient mice. Genes Cells. 2009;8:991–1001.
Tateishi K, Okada Y, Kallin EM, et al. Role of Jhdm2a in regulating metabolic gene expression and obesity resistance. Nature. 2009;7239:757–61.
Okada Y, Scott G, Ray MK, et al. Histone demethylase JHDM2A is critical for Tnp1 and Prm1 transcription and spermatogenesis. Nature. 2007;7166:119–23.
Kuroki S, Matoba S, Akiyoshi M, et al. Epigenetic regulation of mouse sex determination by the histone demethylase Jmjd1a. Science. 2013;6150:1106–9.
Fan L, Peng G, Sahgal N, et al. Regulation of c-Myc expression by the histone demethylase JMJD1A is essential for prostate cancer cell growth and survival. Oncogene. 2016;19:2441–52.
Loh YH, Zhang W, Chen X, et al. Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes Dev. 2007;20:2545–57.
Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;1:277–359.
Cannon B, Nedergaard J. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans). Int J Obes. 2010;34:S7–16.
Mimura I, Nangaku M, Kanki Y, et al. Dynamic change of chromatin conformation in response to hypoxia enhances the expression of GLUT3 (SLC2A3) by cooperative interaction of hypoxia-inducible factor 1 and KDM3A. Mol Cell Biol. 2012;15:3018–32.
Smemo S, Tena JJ, Kim KH, et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014;7492:371–5.
Claussnitzer M, Dankel SN, Kim KH, et al. FTO obesity variant circuitry and adipocyte browning in humans. N Engl J Med. 2015;10:895–907.
Zou Y, Lu P, Shi J, et al. IRX3 promotes the browning of white adipocytes and its rare variants are associated with human obesity risk. EBioMedicine. 2017;24:64–75.
Inagaki T. Regulations of adipocyte phenotype and obesity by IRX3. positive or negative? EBioMedicine. 2017;24:7–8.
Acknowledgements
A summary of this review was presented in the Lilly Award Lecture at the 61st Japan Diabetes Society 2018, Tokyo, Japan. The author would like to express sincere gratitude to Dr. Juro Sakai for his mentoring, his colleagues and collaborators for their helpful support during performing the projects, and Dr. Hiroshi Shibata for critical reading of the manuscript. The author is supported by JSPS KAKENHI (Grant Numbers 18H04796, 17H03631, 25291002), Astellas Foundation for Research on Metabolic Disorders, the Novartis Foundation (Japan) for the Promotion of Science, the Tokyo Biochemical Research Foundation, the Naito Foundation, the Ichiro Kanehara Foundation, Japan Diabetes Foundation, Suzuken Memorial Foundation, and Kao Research Council for the Study of Healthcare Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Ethics policy
This article does not contain any experimental studies with human or animal subjects.
About this article
Cite this article
Inagaki, T. Histone demethylases regulate adipocyte thermogenesis. Diabetol Int 9, 215–223 (2018). https://doi.org/10.1007/s13340-018-0366-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-018-0366-y